FENG et al. — Micronutrient deficiencies and leaf senescence
347
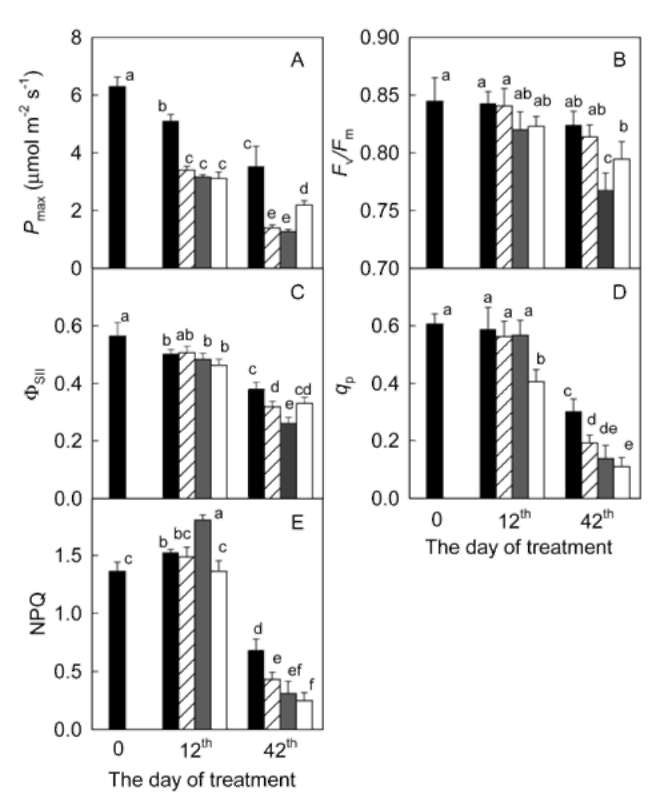
m-2 s-1, 0.7 s). After the fluorescence yield dropped from Fm nearly to F。, "actinic light" source (400 [imol m-2 s-1) was switched on, and the fluorescence yield started to increase. Steady-state fluorescence yield (Fs) was recorded when the fluorescence yield becomes stable. Afterwards, the leaf was again irradiated with a pulse of the saturated beam and the maximum light-adapted fluorescence yield (Fm') was determined. Finally, the minimum light-adapted fluorescence yield (F。') was detected by turning off the "actinic light" and 3 s later switching on the far-red radiation source for 5 s. Maximum photochemical efficiency of PS II was calculated as: FJFm = (Fm - F。)/Fm, quantum yield of PS II photochemistry as: Opsh = (Fm' - Fs) / Fm', photochemical quenching coefficient as: qp = (Fm' - Fs) / (Fm' - Fo'), and non-photochemical quenching coefficient as: NPQ = (Fm/Fm' - 1) (Bilger and Bjorkman, 1990).
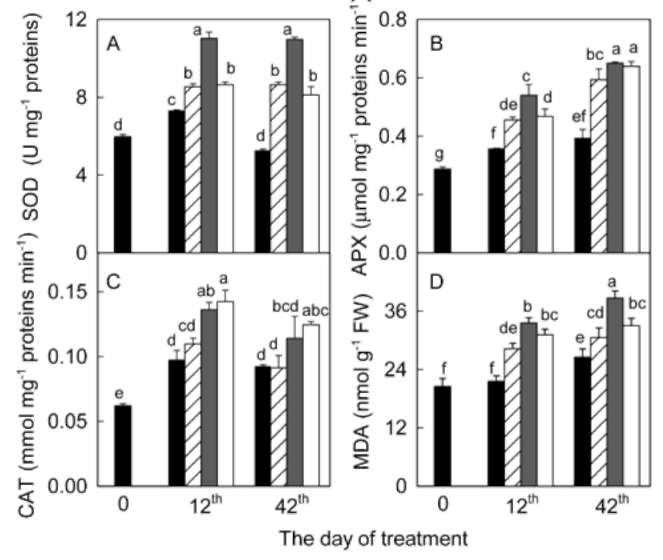
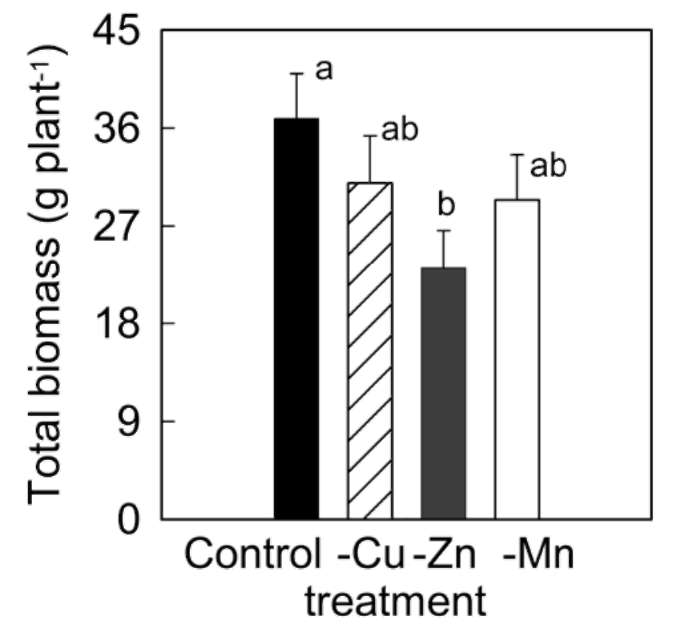
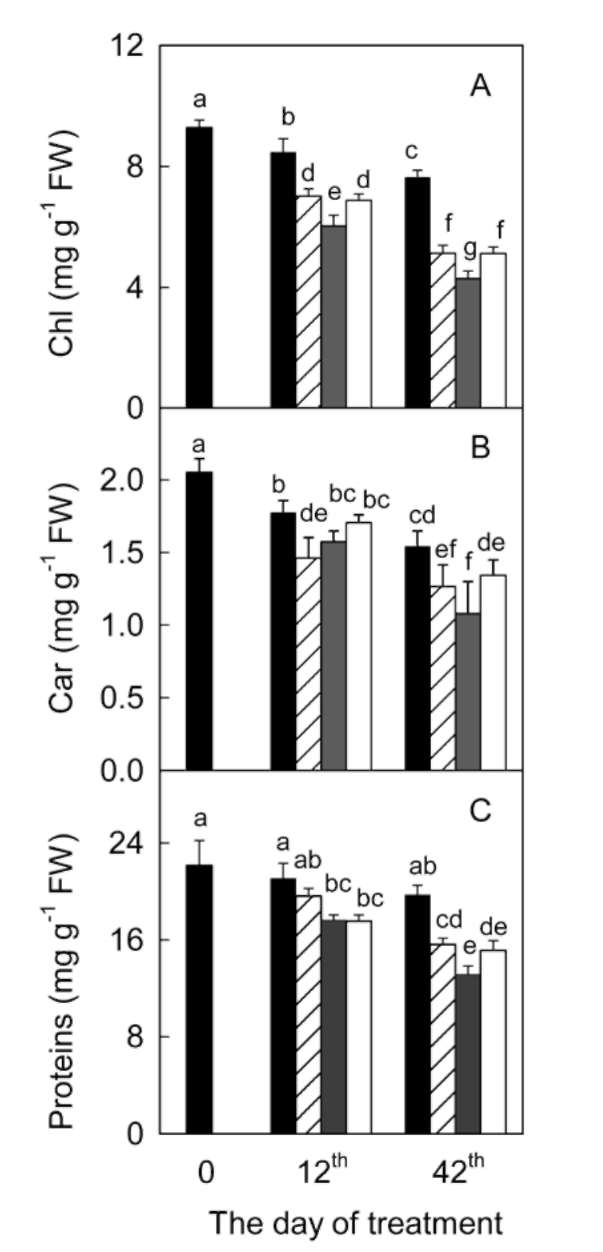
significantly during the 42 days experiment in A. villosum plants grown in full nutrient solution (Figure 1a, b). Mi-cronutrient deficiencies (zinc, copper, and manganese) intensified the decreases in chlorophylls and carotenoids. Micronutrient deficiencies also decreased soluble protein content in A. villosum plants (Figure 1c). Harmful effects of zinc deficiency on this plant were the most serious among the three micronutrients; visible symptoms such as pallid or chlorotic leaves appeared in zinc deficient plants after 40 days of treatment. However, no plant died during the experiment.
Malondialdehyde, soluble protein and chlorophyll pigments. About 0.5 g leaf was homogenized in 10 ml of 10% trichloroacetic acid, and the homogenate was centrifuged at 12,000 xg and 4°C for 10 min. After that, 2 ml 0.6% thiobarbituric acid in 10% trichloroacetic acid was added to an aliquot of 2 ml from the supernatant. The mixture was incubated in boiling water for 30 min, and then quickly cooled in an ice bath. After centrifugation at 10,000 x g for 10 min, the absorbance of the supernatant was determined at 450, 532, and 600 nm, respectively. Malondiadehyde content was calculated according to Hodges et al. (1996). Leaf soluble protein content was determined with the method of Bradford (1976), using bovine serum albumin as a calibration standard. Leaf chlorophyll and caroteniods were extracted with 80% acetone, and were measured with the method of Lichtenthaler and Wellburn (1983).
Antioxidant enzyme activities. Enzymes were extracted by homogenizing sample leaf in 10 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% polyvinylpyrrolidone. The homogenate was centrifuged at 12,000 xg and 4°C for 20 min and the supernatant was used for the following enzyme assays. Superoxide dismutase (EC 1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium at 560 nm (Giannopolitis and Ries, 1977). Ascorbate peroxidase (APX) (EC 1.11.1.11) activ-ity was analyzed by following the decrease in ascorbate at 290 nm (Nakano and Asada, 1981). Catalase (CAT) (EC 1.11.1.6) activity was measured as the decline of the ex-tinction of H2O2 at 240 nm (Aebi, 1984).
Statistical analysis. A one-way ANOVA with a post-hoc test (Duncan test) was conducted to test the differences among treatments in each variable measured in this study. The analysis was carried out using SPPS 13.0 (SPSS Inc., Chicago, Illinois, USA).
Figure 1. Effects of micronutrient deficiencies on the contents of chlorophylls (Chl; a), carotenoids (Car; b), and soluble proteins (c) in leaves of Amomum villosum plants grown at the 12th and 42th days of treatment. Means 士 SE (n = 3). Different letters indicate significantly different at P = 0.05 level. Filled bar, control; hatched bar, copper deficiency; gray bar, zinc deficiency; open bar, manganese deficiency.
RESULTS
Chloroplast pigments and soluble proteins
The contents of chlorophylls and carotenoids decreased