LIN et al. ― Genetic diversity of rice-breeding germplasm in Taiwan
367
All primer sequences and other information for SSRs can be accessed from Gramene (http://www.gramene.org). Four of the 5 STSs with prefixes C, E, and S were obtained from the Rice Genome Research Project (http://rgp. dna.affrc.go.jp, Inoue et al., 1994). The last STS marker, STS208 (F: 5'-CAAAGGTATGATGAGGATAAGG-3', R: 5'-TAGATTCGTCTCGCAGTTTAC-3') was designed as described (Wu et al., 2010). All markers displayed primary polymorphism between indica and japonica (Wu et al., 2010; Lin et al., 2011). PCR reactions and amplification were as described (Lin et al., 2011). The PCR products underwent electrophoresis on 2.5% of Super Fine Resolution (SFR) agarose (Amresco, Solon, Ohio, USA) by Rapid Agarose Electrophoresis (RAGE, Cascade Biologics, Portland, Oregon, USA) in 1x TAE at 250 V for 20 mm. The resolution of the gel system was approximately 5 bp.
locus ranged from 2 to 7, and most markers, 43 (36.13%), revealed 3 alleles. The PIC value for each marker was used to assess the polymorphic level. The mean PIC value for markers was 0.43 with the range of 0.04 (RM6407) to 0.76 (RM481). According to the definition of informative level (Botstein et al. 1980), 35 (29.41%) and 76 (63.87%) mark-ers were highly and reasonably informative, respectively; only 8 (6.72%) were slightly informative. Overall, 119 markers provided sufficient informative polymorphism to evaluate genetic diversity of these 80 varieties.
Analysis of genetic distance
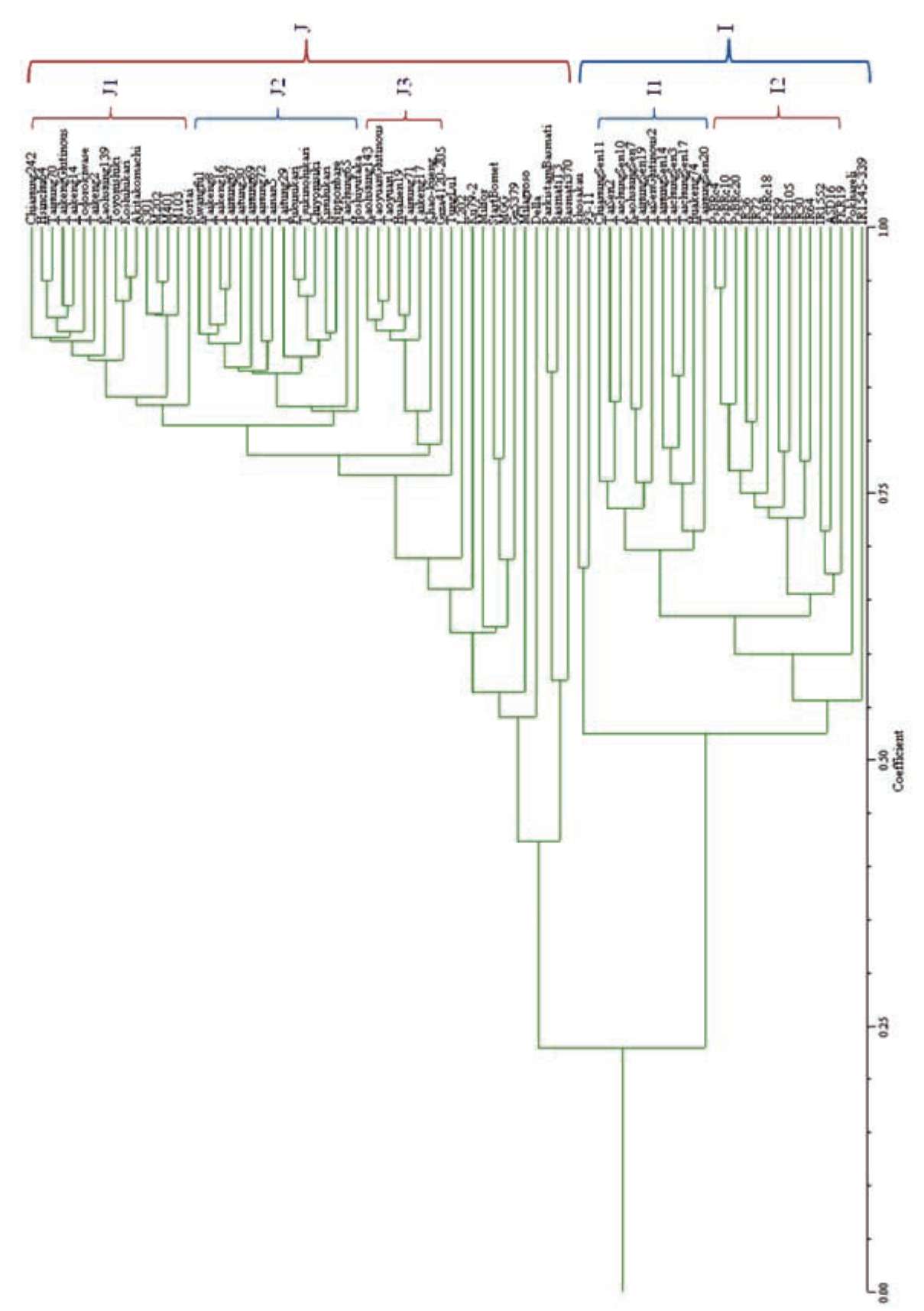
The Dice coefficient was used to assess genetic similarity between two varieties (Dice, 1945), which was consequently subjected to clustering analysis based on UPGMA to construct a dendrogram (Figure 1). The japonica and indica varieties were distinguishably separated into two major different groups with a similarity coefficient of 0.23. Subgroups of japonica and indica varieties further identified by high genetic similarity were classified into 3 clusters (clusters J1 to J3) and 2 clusters (clusters I1 and I2) with a similarity coefficient of 0.78 and 0.63, respectively. However, 12 japonica varieties and 4 indica varieties originated from several countries share great genetic dissimilarity, which were miscellaneous and not easily classified to suitable clusters. According to the Dice coefficient, most japonica varieties, clusters J1 to J3, had greater genetic similarity than did indica clusters (Figure 1).
Data analysis
Because cultivated rice is inbred, one homozygous al-lele is revealed by one DNA band after gel electrophoresis, and one marker represents one locus for the 119 markers used in this study. Presence or absence of a specific allele was indicated as 1 or 0, respectively, and the matrix of 1 and 0 data underwent analysis by polymorphic information content (PIC), genetic similarity and Fst. The PIC was estimated as follows:
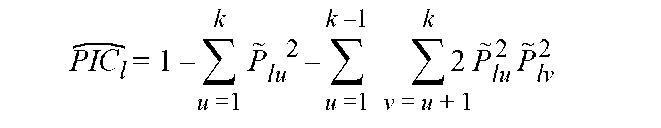
All Taiwan and Japanese japonica varieties were grouped into 3 clusters, clusters J1 to J3, and each cluster contained varieties from these two countries. Koshi-hikari, a leading Japanese variety well known to possess good grain quality, is closely related to Kaohsiung 139 from Taiwan, also with good grain quality. Several varieties released recently, Taikeng 8, Taikeng 16, Taitung 30, Tainung 69, Tainung 72, and Tainan 5, showed high genetic similarity and were grouped together in cluster J2 with Tainung 67, an elite germplasm that has undergone modern rice breeding (Figure 1). Tung Lu 1, an old upland variety known as tropical japonica, showed little genetic similarity to the other temperate japonica varieties by UPGMA analysis and was not grouped into the other three major japonica clusters. Varieties introduced from the United States, such as M103, M202, M401, and S301, share a genetic background with a similarity coefficient of 0.91 and were clustered together in cluster J1 (Figure 1). Most varieties introduced from the other countries exhibited larger genetic distance than those introduced from Japan and the United States, which were classified as in japonica group but not easily separated as distinct clusters (Figure 1). Nevertheless, three aromatic varieties, Pakistan Basmati, Basmati T3, and Basmati 370, were in the same cluster, which was distinguished from the other japonica varieties.
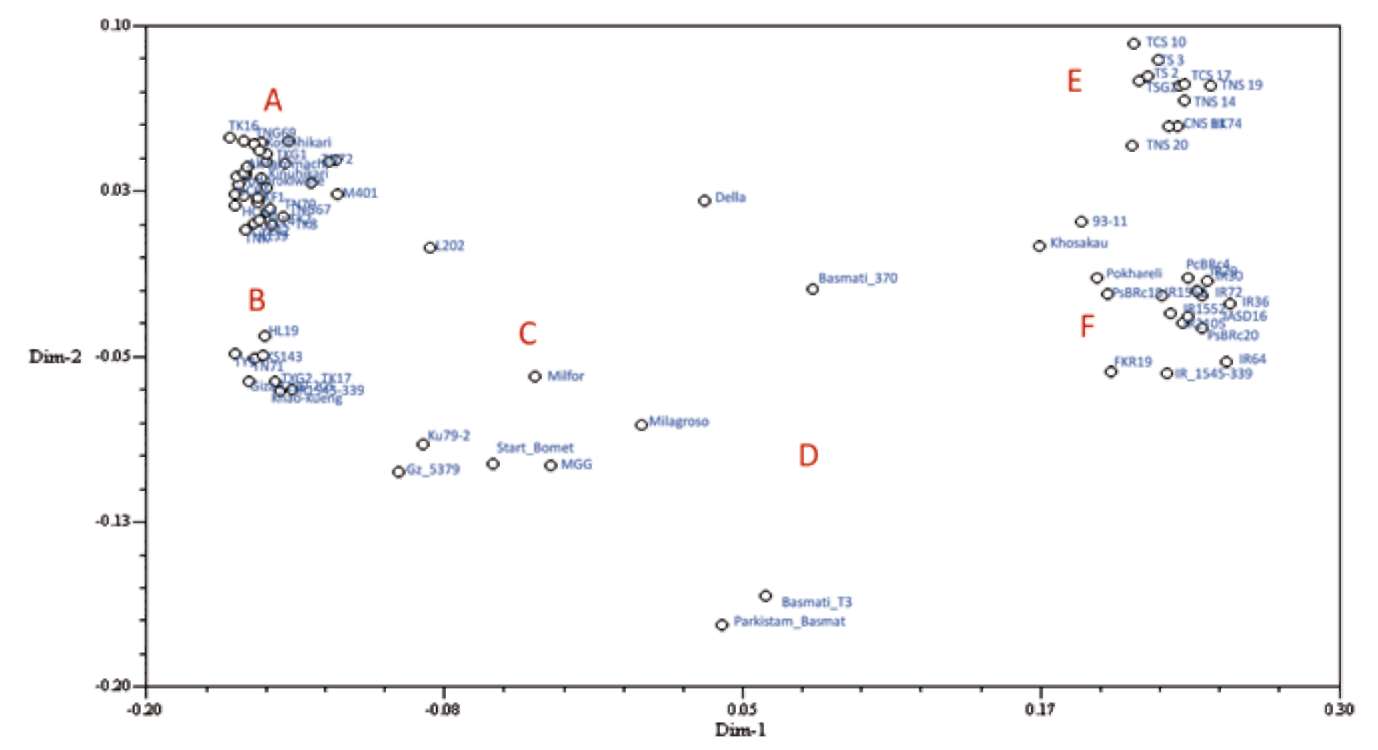
where l is locus; k is the number of alleles of locus l; Plu is the frequency of allele u; Plv is the frequency of allele v (Botstein et al., 1980). The PIC value for each marker was used to justify the polymorphic information, and the mean PIC value for a group of individuals implies the genetic diversity within the group. Both the PIC for each marker and mean PIC for each group were determined by use of Pow-erMarker (Liu and Muse, 2005). Genetic similarity and dissimilarity were evaluated by modified Rogers' distance (Goodman and Stuber, 1983). We used sequential agglom-erative hierarchic non-overlapping (SAHN) clustering and then unweighted pair-group method with arithmetic mean (UPGMA) dendrogram to infer genetic relationships and construct a phylogenetic tree. Decnter and Eigene modules were used to transform and calculate two dimension of principle coordinate analysis (PCoA, Gower, 1966). UPG-MA and PCoA analysis involved use of NTSYS-pc v.2.21 (Rohlf, 2005).
RESULTS
Polymorphic levels of molecular markers
Using 114 SSR and 5 STS markers to analyze the genetic diversity of 80 modern cultivars and germplasm used in Taiwan rice-breeding programs, we found 395 alleles with a mean of 3.5 alleles (Table 2). The allele number per
All 28 indica varieties formed the indica group with 2 distinct clusters, clusters I1 and I2 (Figure 1). All Taiwan indica varieties shared high genetic similarity with the oth-