ZHANG et al. ― Community responses to heterogeneity
379
the experiment was set up. Despite this defect, we still could address the questions raised in the introduction as long as we treated the two species as one group. For each plant group, we counted number of nodes and measured total shoot length. Then the plants in each group were harvested, oven-dried at 70°C for at least 48 h and weighed.
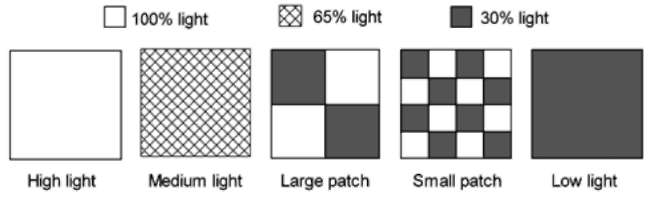
Figure 1. Schematic representation of the experimental design. The experiment consisted of three homogeneous treatments (High light - the whole community in the container received 100% light in the greenhouse; Medium light - the whole community received 65% light; Low light - the whole communities received 30% light) and two heterogeneous treatments (Large patch - the whole community was divided into four large patches; two patches received 100% light and the other two 30% light. Small patches - the whole community was divided into 16 small patches; eight patches received 100% light and the other eight 30% light). The light received by the whole communities in the two patchy treatments was the same as that in the homogeneous medium light treatment.
Data analyses
Biomass, number of nodes and shoot length of each plant group were collected and the data of the three plant groups were summed up as the values of the communities. The data were statistically tested for differences by the analysis of variance (one-way ANOVA) followed by five planned comparisons (Sokal and Rohlf, 1995) using the Contrast options of SPSS (SPSS, Chicago, IL, USA). The first three contrasts (i.e., high vs. medium light treat-ment, high vs. low light treatment and medium vs. low light treatment) tested the effects of light intensity under homogenous treatments on the three growth variables of the communities as well as on those of each plant group. The fourth contrast [i.e., medium light treatment vs. (large and small patch treatment)] tested the overall effects of light heterogeneity on the growth variables, and the fifth contrast (i.e., large vs. small patch treatment) examined the effects of the patch scale. All analyses were conducted with SPSS 16.0 software (SPSS, Chicago, IL, USA).
net (Figure 1). The two heterogeneous light treatments were coded as large patch treatment (each box consisted of four large patches) and small patch treatment (each box having 16 small patches), respectively (Figure 1). The shading net that allows 30% light to pass through (used in the homogeneous low light treatment) was used in both patchy treatments. In the large patch treatment, the shading net covering the top of each box was divided into four 17 cm x 17 cm patches, and in two patches the shading net was removed so that 100% light could pass through these two patches. In the small patch treatment, the shading net covering the top of each box was divided into 16 8.5 cm x 8.5 cm patches, and in eight patches the shading net was removed so that 100% light could pass through these patches (Figure 1). Therefore, the total amount of light received by the whole communities in the two patchy treatments was the same as that in the homogeneous medium light treatment.
RESULTS
Effects at community level
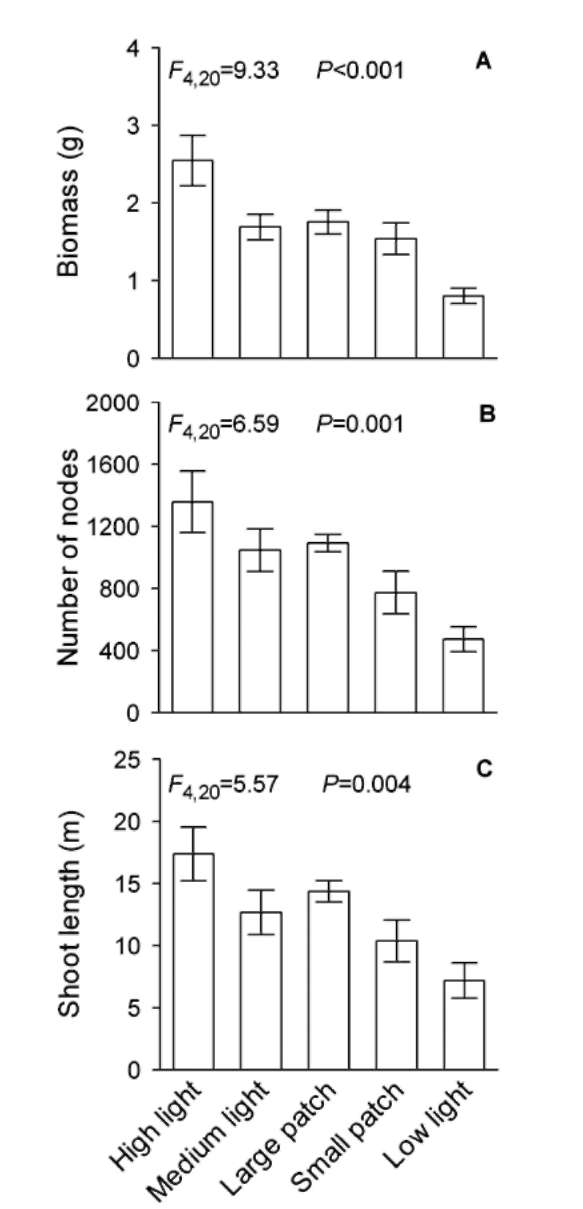
Under homogeneous treatments, decreasing light intensity significantly decreased total biomass, total number of nodes and total shoot length of the communities (Figure 2, Table 1). However, total biomass, total number of nodes or total shoot length did not differ significantly among the three medium light treatments (i.e., the homogeneous medium light treatment, the large patch treatment and the small patch treatment; Figure 2, Table 1), suggesting that light heterogeneity did not affect these three variables of the community.
The experiment lasted ten weeks and ended on 22 October 2010. During the experiment, water was added to each box every four or five days to compensate for the loss by evaporation, and water in each box was also partly replaced three times to renew its quality. In the greenhouse the temperature was 21.8 ± 0.1°C and relative humidity 77.0 ± 0.3% (mean ± SE; measured hourly by two Hygrochron temperature/humidity loggers, iButton DS1923; Maxim Integrated Products, USA).
Effects at species level
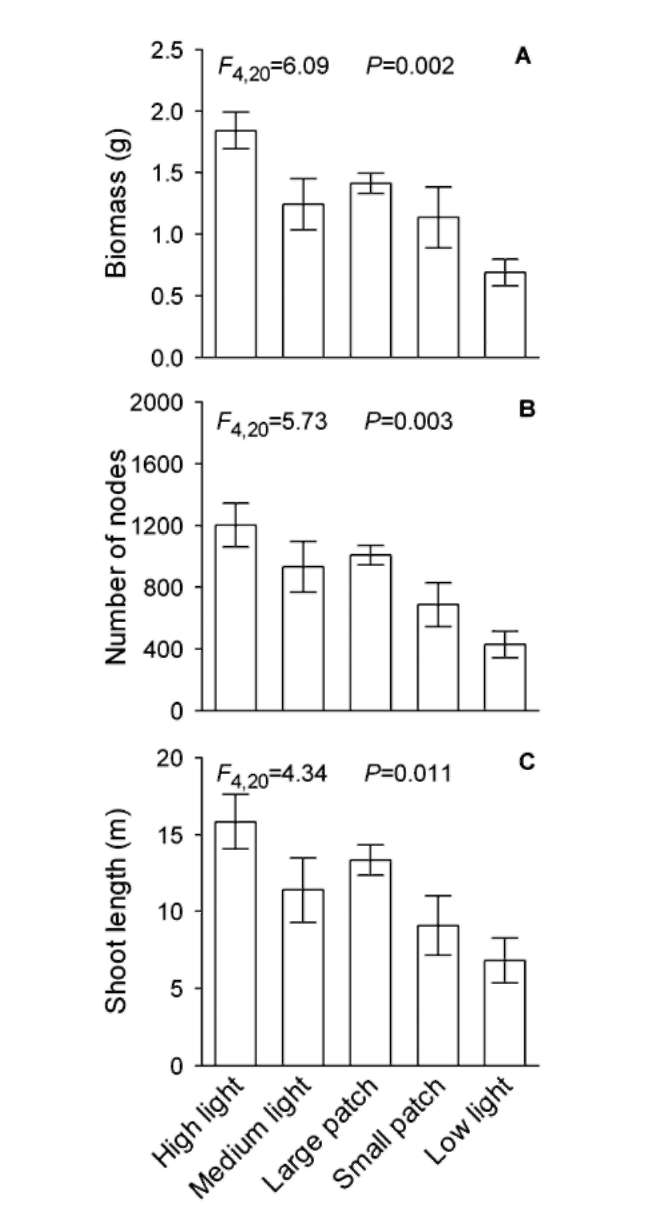
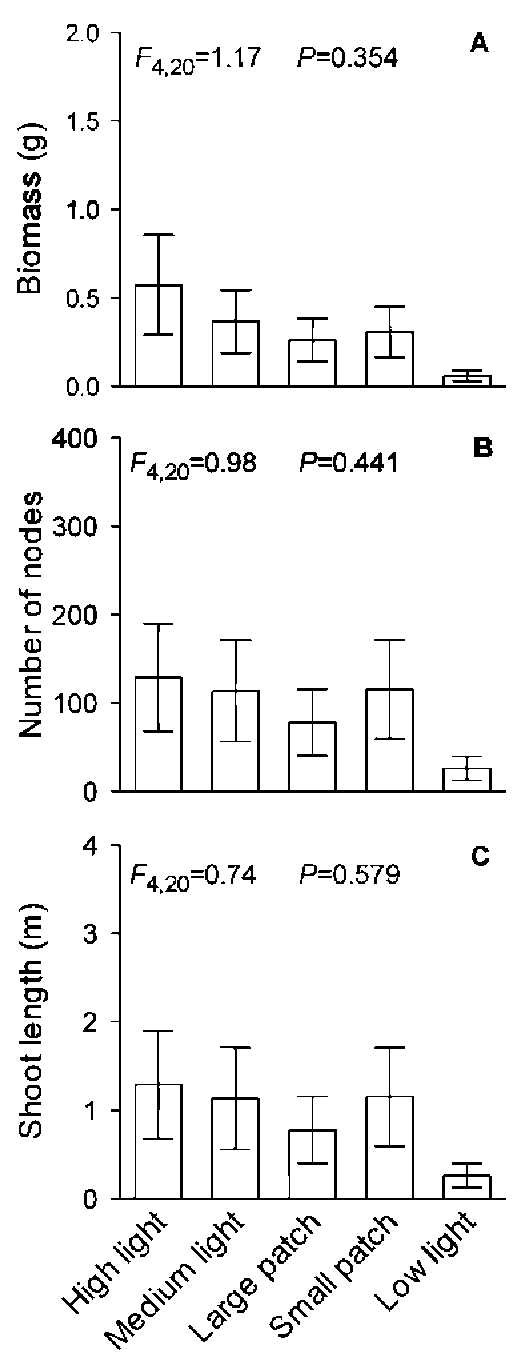
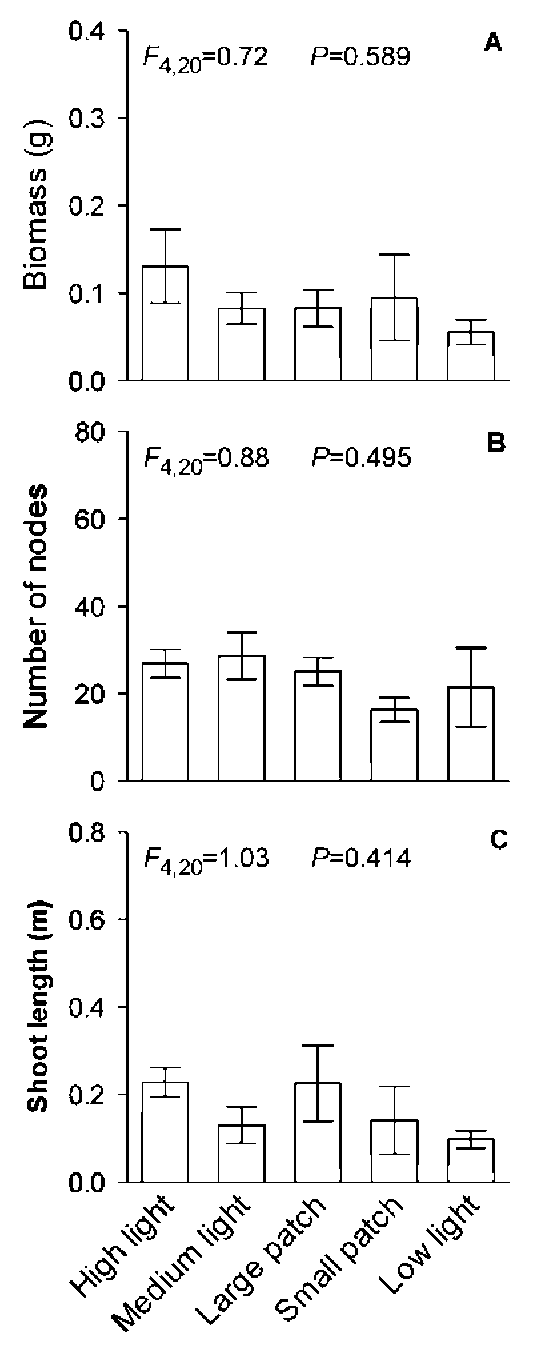
Under homogeneous treatments, total biomass, total number of nodes and total shoot length of the H. verticil-lata - E. densa group decreased greatly with decreasing light intensity (Figure 3, Table 1). However, none of the three variables of the H. verticillata - E. densa group differed significantly among the homogeneous medium light treatment, the large patch treatment and the small patch treatment, suggesting that light heterogeneity had no effect on these three parameters of the H. verticillata - E. densa group (Figure 3, Table 1). Neither C. demersum nor M. verticillatum showed significant differences in total biomass, total number of nodes or total shoot length among the five light treatments (Figures 4 and 5, Table 1), suggesting neither light intensity nor spatial heterogeneity affected growth of these two macrophytes.
Harvest and measurements
On 22 October 2010, the surviving plants in each box were harvested and sorted into three groups, i.e., (1) M. verticillatum, (2) C. demersum and (3) the two species in the Hydrocharitaceae family (H. verticillata and E. den-sa). We combined plants of H. verticillata and E. densa because at the end of the experiment it was rather difficult to precisely distinguish and separate plants of H. verticil-lata from those of E. densa, which was unexpected when