460
Botanical Studies, Vol. 53, 2012
MATRIALS AND METHODS
Chemicals, reagents and apparatuses
The potassium salt of atractyloside (>99% by TLC), two internal standards (5a-androstane-3p,17p-diol and cholesterol) and the derivative reagent, trimethylsilyl imi-dazole (TMSI), were purchased from Sigma Chemical-Aldrich (ST. Louis, MO, USA). Ethyl acetate, methanol and pyridine (Silylation grade) were obtained from Merck (Darmstadt, Germany). All chemicals were analytic or ultrapure grade and used without purification. The water used was the ultrapure deionised water prepared by Milli-Q filtration. The standard solutions of ATR were 100 and 1000 |ig/ml, and stored in the dark at -20°C. Hyper-Sep C18 SPE cartridge (200 mg bed weight /3 ml volume) were purchased from Thermo Scientific Corp. (USA). A 12-port vacuum manifold was used for all extractions.
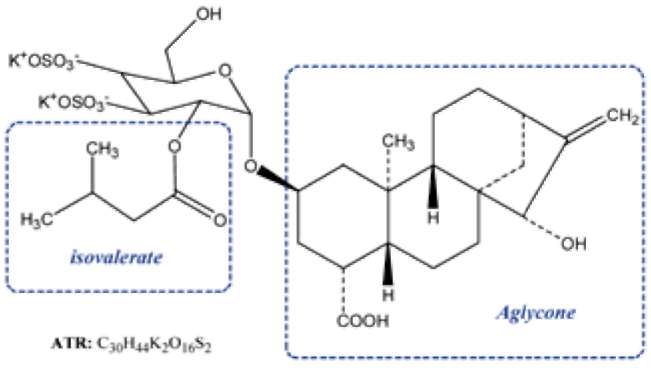
Figure 1.
The chemical structures of atractyloside (R= H) and
corboxy-atractyloside (R= COOH).
(Duan et al., 2008; Wang et al., 2008).
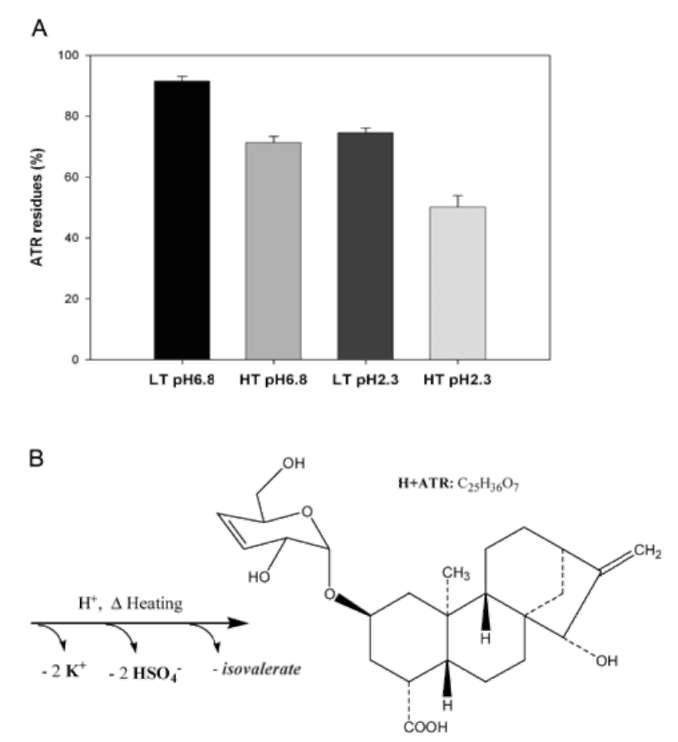
The toxicity and biochemistry of ATRs have been reviewed (Obatomi and Bach, 1998; Daniele et al., 2005). ATR specifically binds to adenine nucleotide translocation in the inner mitochondrial membrane and competitively inhibits ADP and ATP transport. The complete mechanism of toxicity of ATR is not fully understood. However, crude herbal medicines are brewed (infused and extracted with hot water) before drinking. The toxicity and chemical profiles of ATRs are highly dependent on the variations of both species and processing (Obatomi and Bach, 1998; Steenkamp et al., 2004; Daniele et al., 2005).
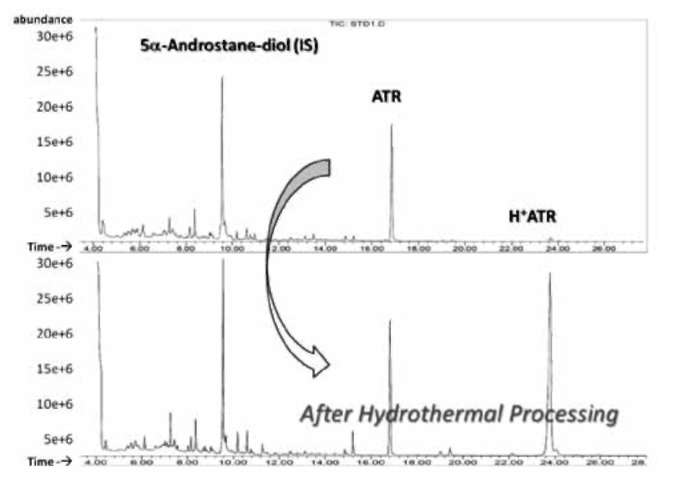
Stability of ATR in processing
Five 200 ml aliquots of 100 [ig/ml ATR were prepared to evaluate the kinetic stability of ATR by heating and acidification. One aliquot was untreated and stored at ambient temperature (about 22°C) as the negative control. The other samples were adjusted to pH 6.8 and pH 2.3 in 100mM phosphate buffered saline (PBS), and the solutions were incubated at 98°C and 65°C, respectively. Three 2 ml samples were collected after heating for 120 minutes. Then, the samples were extracted using SPE and deriva-tized for GC/MS analysis.
Gas chromatography-mass spectrometry (GC/MS) is widely used in forensic, doping, and toxic screening laboratories because it has the advantage of high sample throughput, rapid detection, and economic feasibility (Lau-rens et al., 2001; Hsiao, 2007; Chen et al., 2011). The aim of this work was to develop a rapid method for determination of ATR in TCMs by GC/MS. Also, the quantitative data acquired will help us to assess the safety and risk of medicinal herbs in pharmaceutical industries and ethnop-harmacology (Chau and Wu, 2006; Hsiao, 2007; Han et al., 2009; Jiang et al., 2010).
Sample preparation and aqueous infusion
The rhizomes of the A. macrocephala and A. lancea and the fruits of Xanthium strumarium were harvested in the greenhouse of Institute of Plant Biology in NTU when the active principles are believed to reach the highest. After cleaning and drying, the specimens of herbs were pulverized to a powder before the infusion process (hot water extraction). Five grams of pulverized powder and 40 ml of water were added into a 250 ml round bottomed flask and heated to boiling for 15 min. Then, the hot solution was

Figure 2.
The Asteraceae plants used in TCM.