470
Botanical Studies, Vol. 53, 2012
Table 1.
The incidence of fruit rot disease and occurrence frequency of Botryosphaeriaceae species on mango fruits exhibiting
symptoms of fruit rot during 2009-2011.
|
Region
|
Year
|
Incidence (%)a
|
|
Frequency of occurrence (%)b
|
|
|||
|
Lasiodiplodia theobromae
|
Neofusicoccum parvum
|
Neofusicoccum mangiferae
|
Fusicoccum aesculi
|
|||||
|
Fanshan
|
2009
|
20.1
|
1.8
|
16.1
|
28.6
|
12.5
|
||
|
|
2010
|
18.7
|
6.4
|
25.5
|
4.3
|
34.0
|
||
|
|
2011
|
22.5
|
0.0
|
37.8
|
20.0
|
37.8
|
||
|
Yujing
|
2009
|
35.0
|
9.7
|
3.2
|
3.2
|
25.8
|
||
|
|
2010
|
25.6
|
2.8
|
30.6
|
0.0
|
47.2
|
||
|
|
2011
|
23.8
|
10.5
|
26.3
|
7.9
|
13.2
|
||
|
Guntain
|
2009
|
46.0
|
7.5
|
0.0
|
2.5
|
17.5
|
||
|
|
2010
|
53.8
|
3.3
|
5.0
|
0.0
|
8.3
|
||
|
|
2011
|
58.1
|
13.1
|
3.6
|
2.2
|
12.4
|
||
aIncidence of mango fruit rot in each area was calculated by the following formula: Disease incidence (%) = (Number of fruits which showed only symptoms of fruit rot but not anthracnose/Total number of fruits) x 100%.
^Frequency of occurrence (%) = (Number of fruits colonized by a pathogen/Total number of fruits with fruit rot symptoms) x 100%.
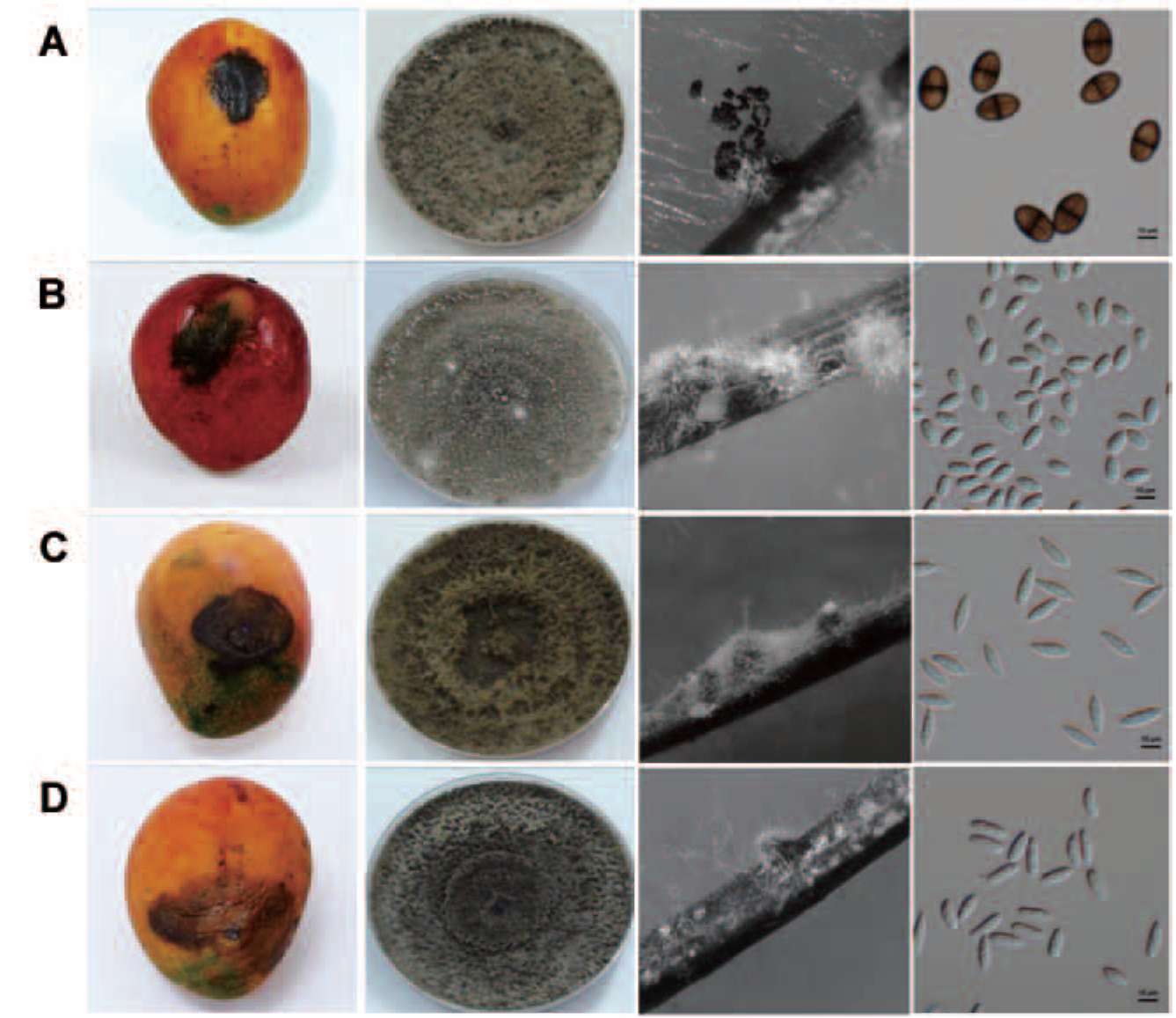
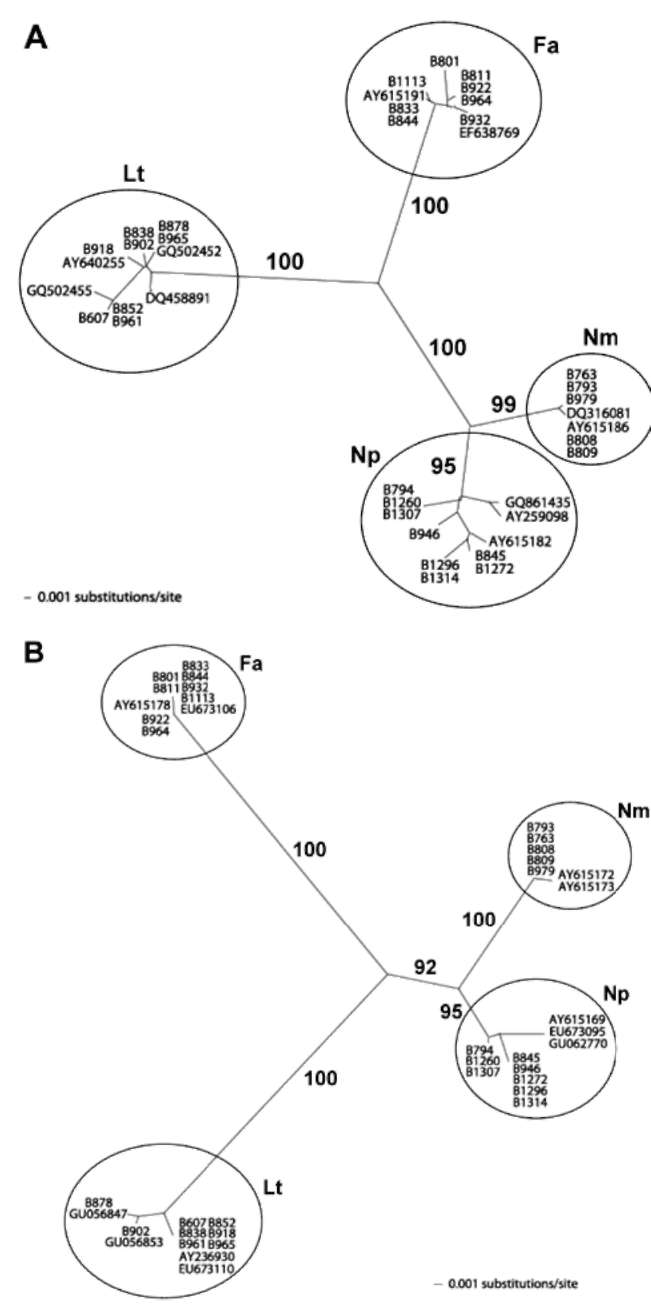
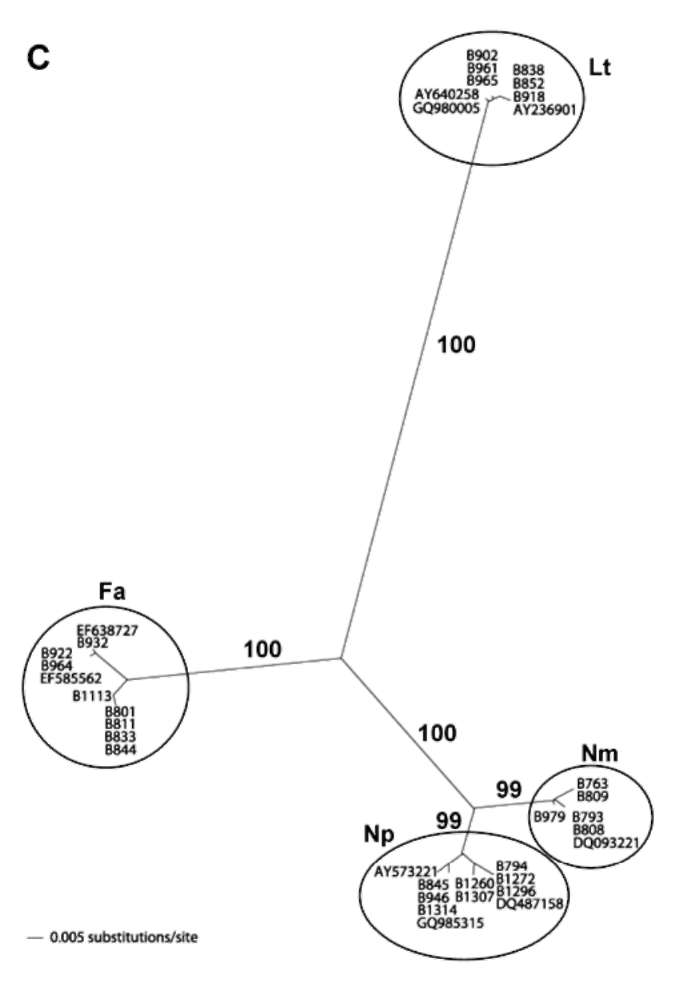
Figure 1.
Symptoms of mango fruit rot and morphology of fungal colony, pycnidia, and conidia. A: Lasiodiplodia theobromae; B:
Neofusicoccum mangiferae; C: Fusicoccum aesculi; D: N. parvum. Bar = 10 μm.