480
Botanical Studies, Vol. 53, 2012
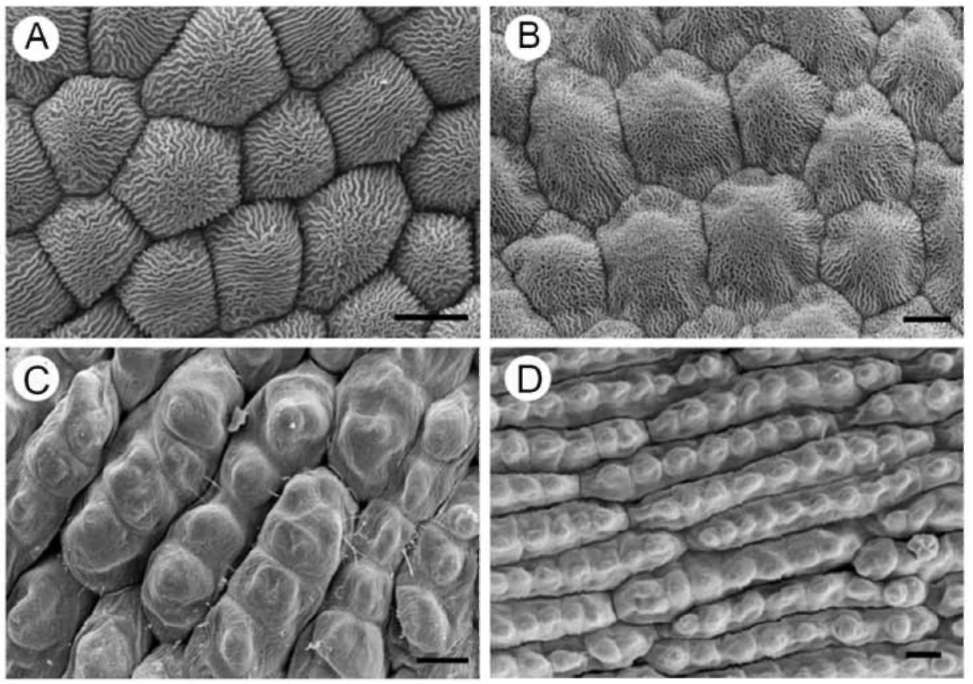
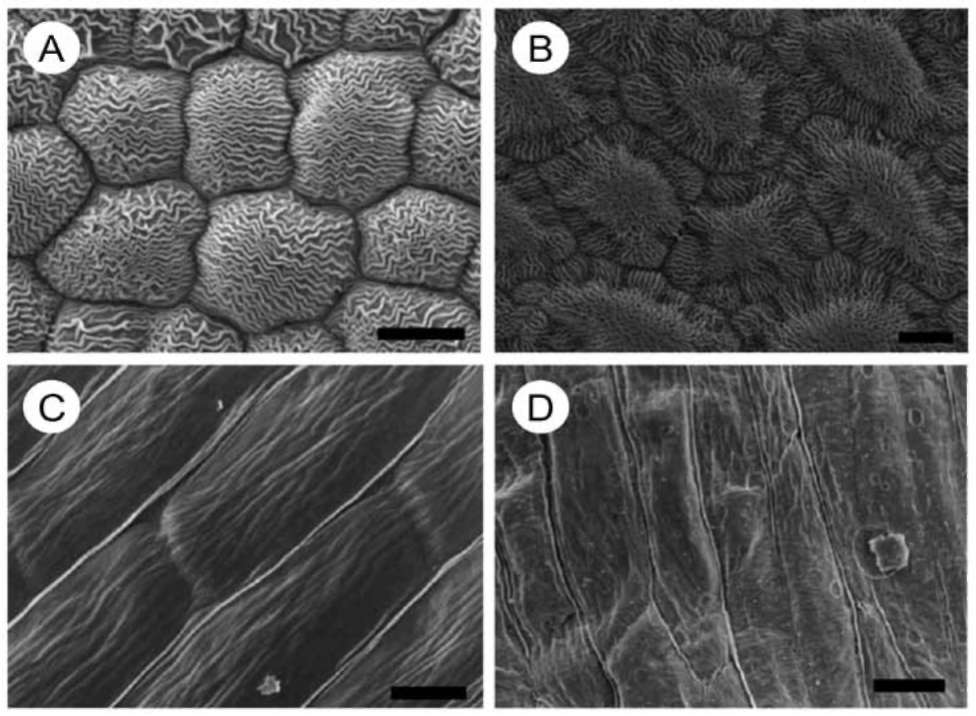
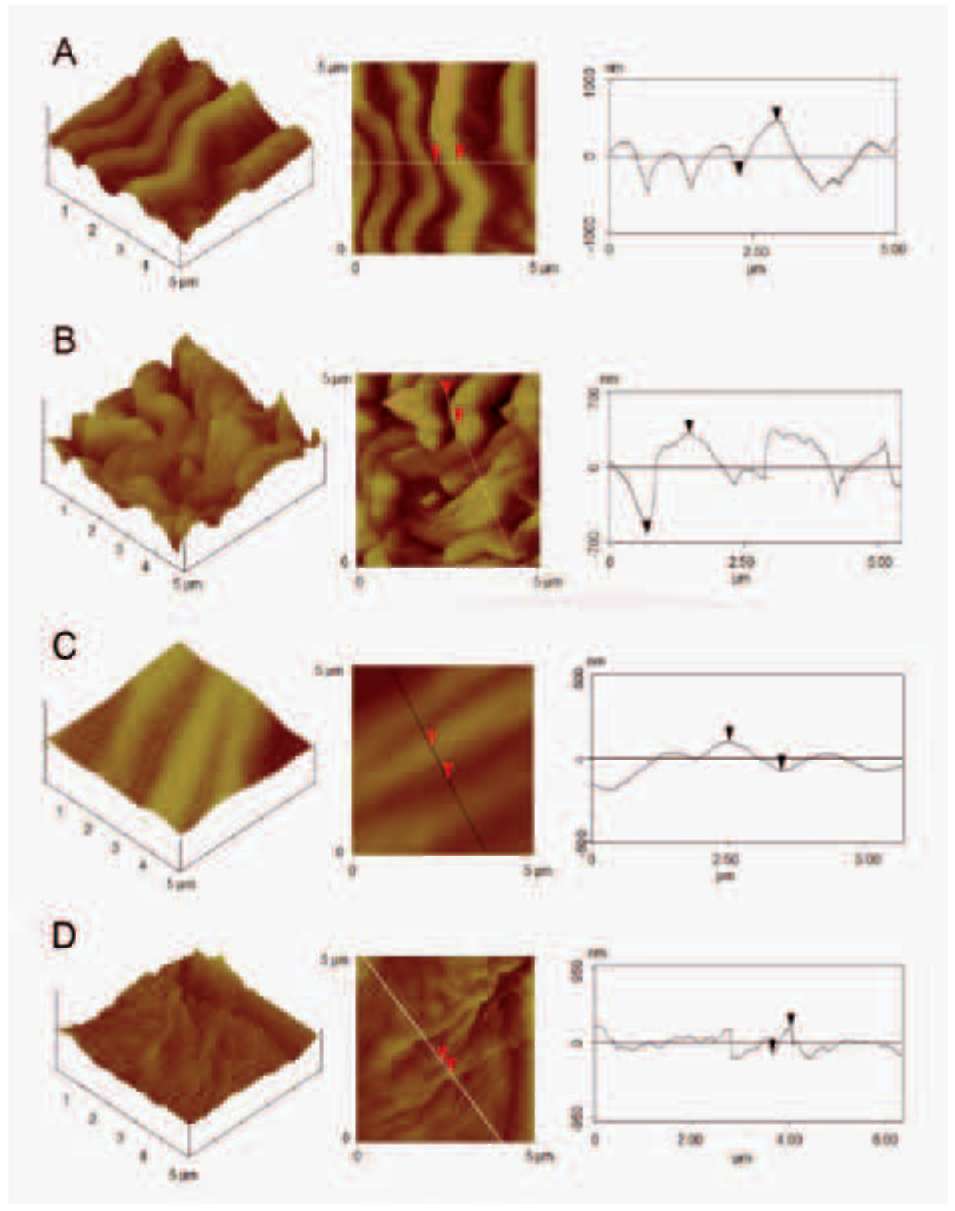
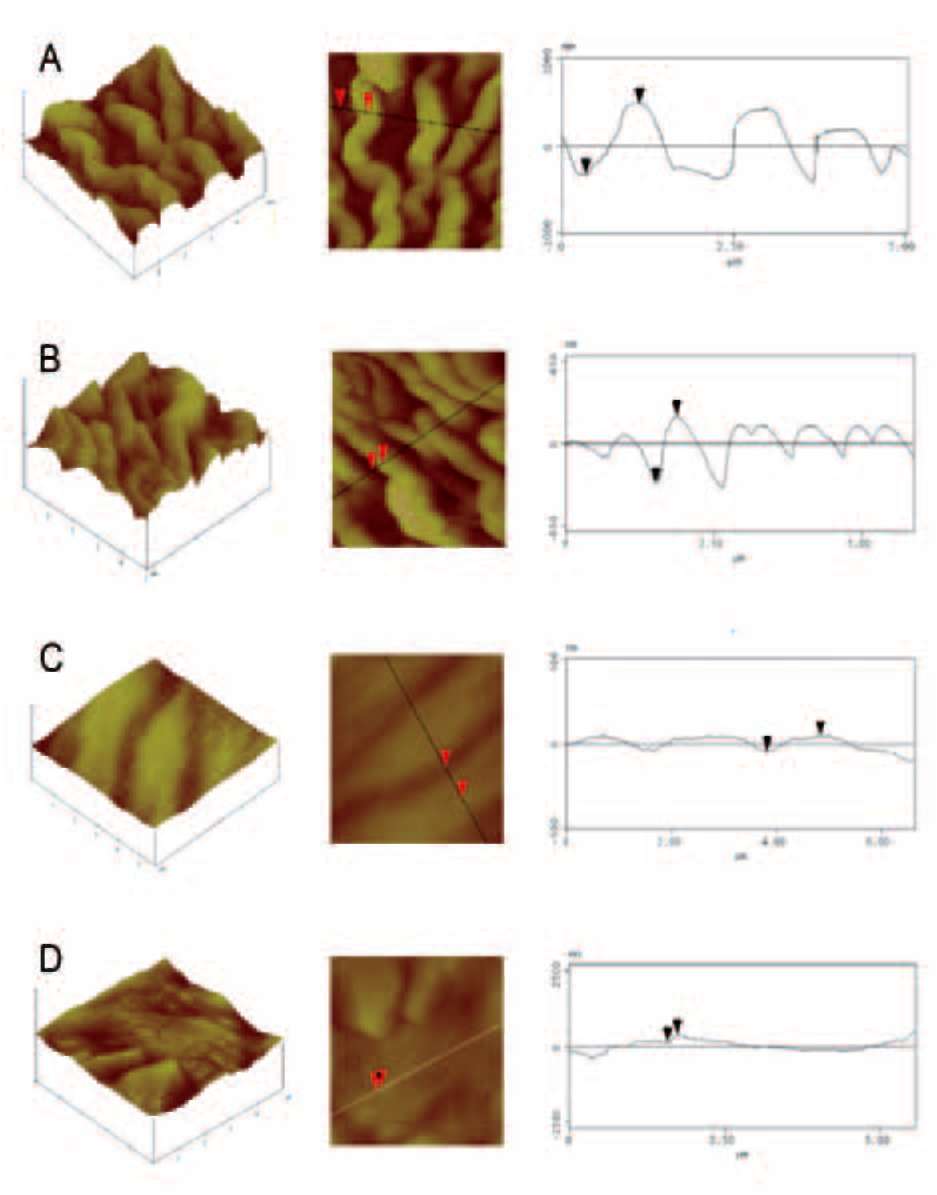
ing species, consistent floristic elements of the Mediterranean landscape. Light microscope, scanning electron microscope (SEM) and atomic force microscope (AFM) were used to study the topography of the adaxial and the abaxial petal surface. Scientific work has demonstrated the suitability of SEM and AFM, for observations of structural traits in leaves (Mechaber et al., 1996; Koch et al., 2004; Solga et al., 2007; Agrawal et al., 2009) and petals (Kay et al., 1981; Gale and Owens, 1983; Kaplan, 2008; Whitney et al., 2009; Argiropoulos and Rhizopoulou, 2012). High-resolution imaging using AFM reveals hierarchical micropapillae and striated nanosculptures that increase the size of surface area of petal epidermises, and influence optical and adhesive properties of the delicate tissues (Miller et al., 2011; Rands et al., 2011; Argiropoulos and Rhizopoulou, 2012; Chimona et al., 2012). To the best of our knowledge, structural and functional properties of the petals' surfaces of the examined species (cited alphabetically) Cistus creticus, Cistus salviifolius, Eruca sativa, and Sinapis arvensis, using high resolution imaging at the nanometer scale, which may greatly expand our understanding about the microsculpture of the delicate tissues, have not been hitherto reported.
MATERIALS AND METHODS
Plant material
The study was carried out at the Campus of the University of Athens in Greece (38。 57' N, 23。 48' E, altitude 250 m). Expanded, turgid flowers were harvested from four plant species that grow in an open field and are presented here according to the succession of their flowering period: A) Sinapis arvensis L., Cruciferae (Figure 1A). B) Eruca sativa (Miller) Thell., Cruciferae (Figure 1B). C) Cistus creticus L. (C. incanus subsp. creticus), Cistaceae (Figure 1C). D) Cistus salviifolius L., Cistaceae (Figure 1D). Flowering was observed on a regular basis, every day during the blossoming period, of the above mentioned species. Flowers of S. arvensis and E. sativa exhibit a three-day life span, while those of C. creticus and C. salviifolius are ephemeral, by exhibiting one-day floral span. Sampling was made at the end of March 2009 and 2010. The above mentioned species begin to bloom in the end of February, when some appearance of spring is seen; their flowering period coincides with a monthly precipitation that varies from 70 mm (February) to 45 mm (March), while the average monthly temperature varies between 10。C and 15。C, respectively.
Microscopy
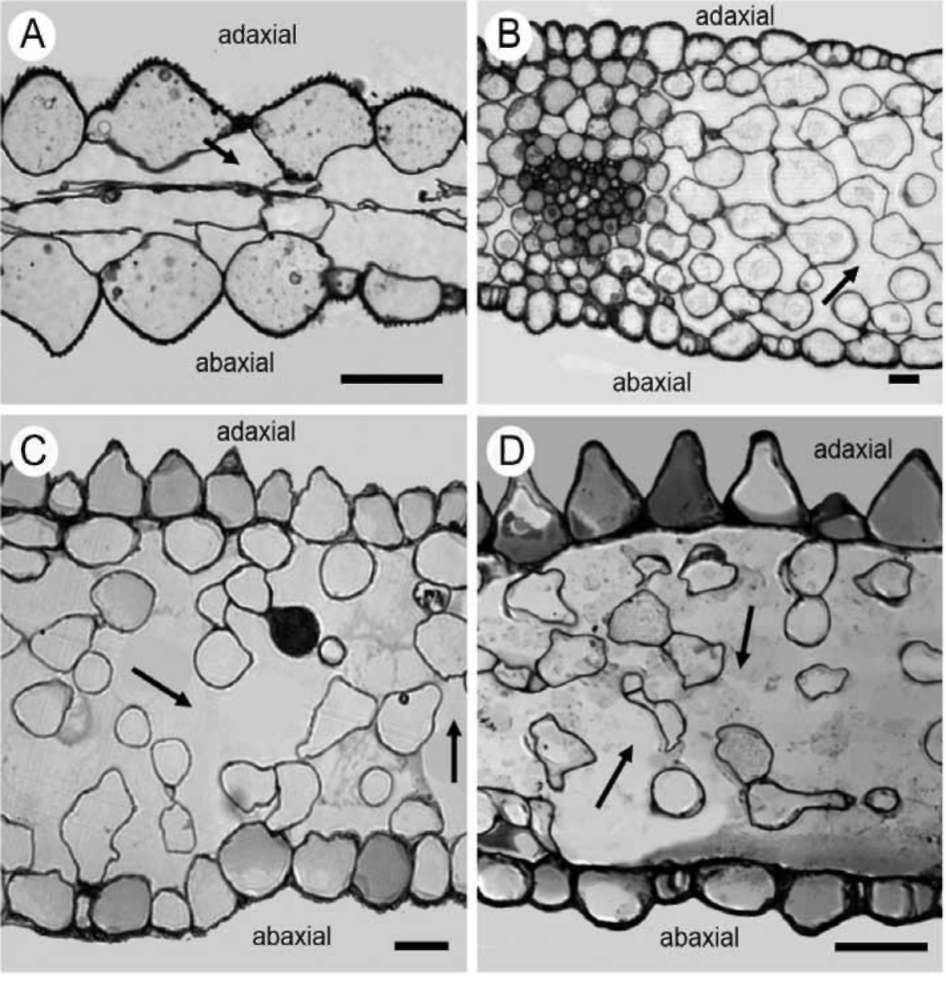
The study was carried out in developed petal regions (Figure 1). Samples from the petal blade were carefully cut in square pieces (4 mm2) and fixed in 3% glutaraldehyde in Na-phosphate buffer at pH 7, at room temperature, for 2 h. Plant material was washed three times by immersion in buffer for 30 min each time; then, it was post fixed in 1% OsO4 in the same buffer at 4°C and dehydrated in acetone
solutions. Dehydrated tissues were embedded in SPURR (Serva) resin. Semi-thin sections of resin-embedded tissue (LKB Ultratome III microtome) were stained in Toluidine Blue '0', in 1% borax solution, photographed and digitally recorded using a Zeiss Axioplan light microscope (Carl Zeiss Inc., Thornwood, N.Y.) equipped with a digital camera (Zeiss AxioCam MRc5). Dehydrated samples were dried at the critical point in a Bal-tec CPD-030 dryer, mounted with double adhesive tape on stubs, sputter coated with 20 nm gold in a Bal-tec SCP-050. The adaxial and abaxial epidermises of petals were viewed using the scanning electron microscope JEOL JSM 840 (JEOL Ltd, Tokyo, Japan). Also, the adaxial and the abaxial petal areas (25 (im2) were imaged by using a tap mapping atomic force microscope (Multimode SPM; Veeco, Santa Barbara, CA, USA). Several parameters were analysed and processed, using the software package Nanoscope III (Veeco, USA), in order to detect detailed information for the surfaces of petals. The quantitative measurements include surface roughness (Ra) of the tissues, horizontal and vertical distances that represented the height of a step between nanofolds, and the length between the markers that represented the surface distance. The surface area ratio (Sr), representing the density of microfolding, was the percentage of the three-dimensional surface, compared to the projected flat surface area, on the threshold plane. Angles (。) between a straight line connecting the cursors and the horizontal surface were measured on both petal surfaces
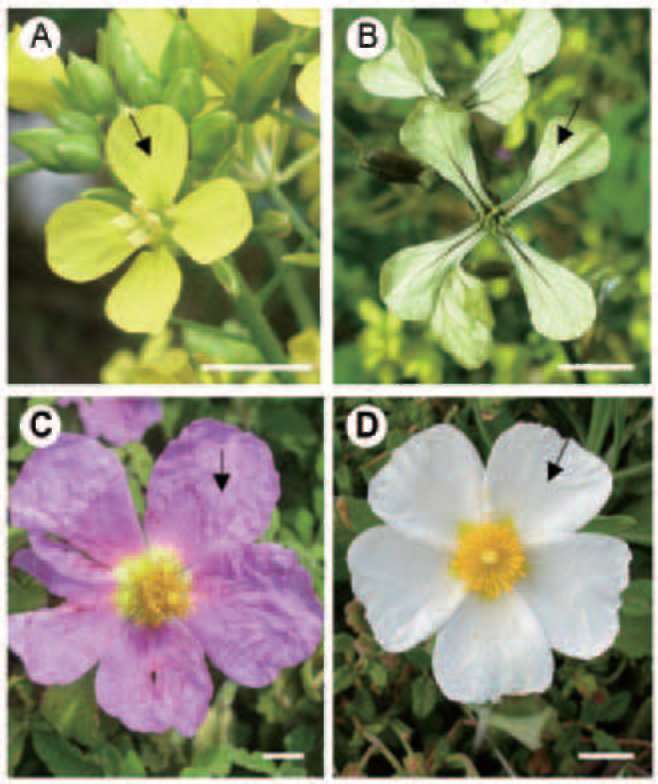
Figure 1. Flowers of S. arvensis (A), E. sativa (B), C. creticus (C) and C. salviifolius (D); sampling regions of adaxial, fully expanded petal tissues are indicated by arrows. Scale bars: 1 cm.