HUANG et al. ― Floral biology of Bidens pilosa var. radiata, an invasive plant in Taiwan
503
of the same individual was used to represent the value of that individual, and five individuals were sampled.
Number of pollen grains and pollen/ovule ratio
To estimate the number of pollen grains and P/O ratio, pollen grains of a single tubular floret was counted with a counter chamber under a microscope. Pollen numbers of two florets from one individual were counted and means were calculated to represent the pollen number of this individual. In total, 20 florets from 10 individuals were counted. The difference of number of pollen grains among 10 individuals was analyzed with ANOVA (general linear model procedure of SAS, release 9.1, SAS Inst. Inc.).
RESULTS
Number of disk florets/capitulum
Among the 58 capitula counted, most (50) capitula had 35 to 55 disk florets, only 5 capitula had disk florets less than 35 and 3 capitula had disk florets more than 55 (Figure 1). The mean florets number of each capitulum was 44.1 士 1.0 (mean 士 s.e., n = 58), and there was significant difference in the number of disk florets per capitulum among the ten individuals (〜48 = 2.11, p = 0.047).
Floral morphology
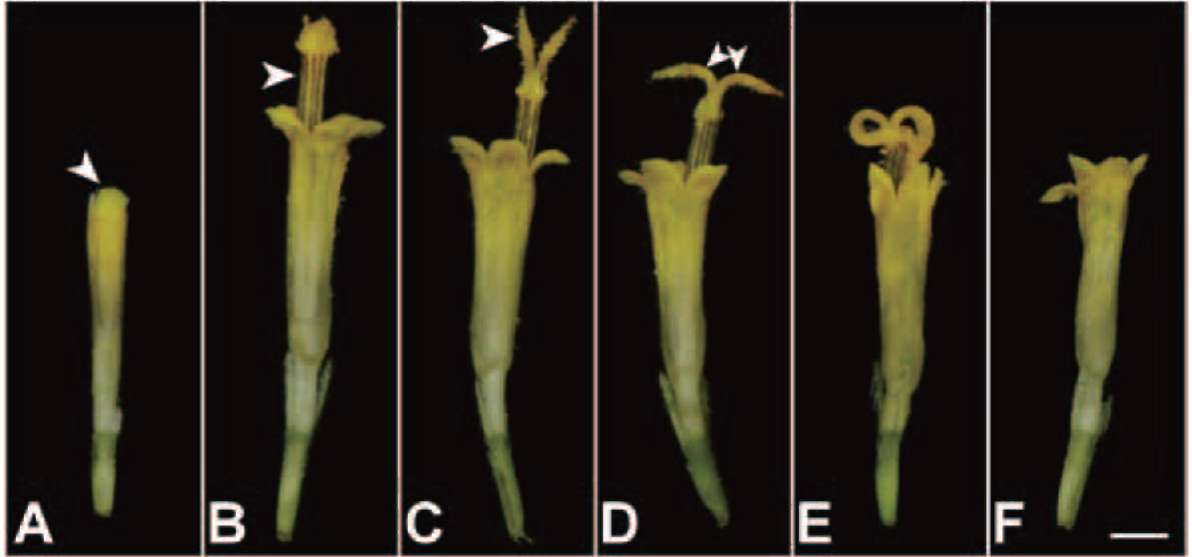
The dissection of a disk floret of B. pilosa var. radiata is shown in Figure 2A. A disk floret was composed of five joined petals (forming a tubular corolla), five stamens with coherent brown anthers (forming an anther tube) and separated white filaments (Figure 2Bb 2B2), a long style with two branches at the apex ascending from the center of the anther tube and an inferior ovary.
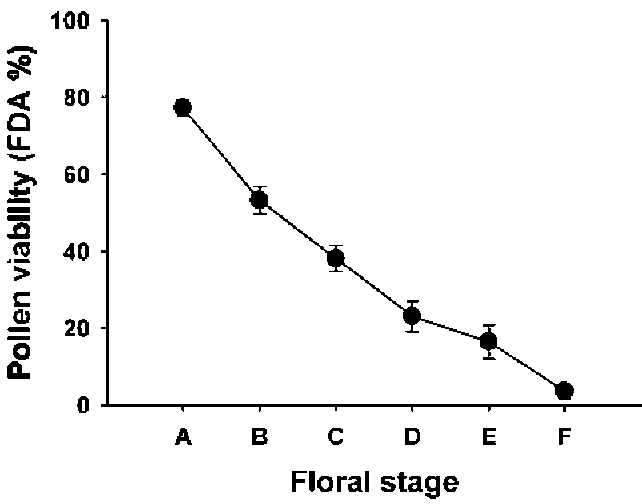
Before disk floret anthesis, the anthers dehisce and release of pollen grains into the anther tube (Figure 2B1). The pollen grains (Figure 2C), diameter of 32.1 士 0.5 fim (with spinule) or 27.9 士 0.8 fim (without spinule), were
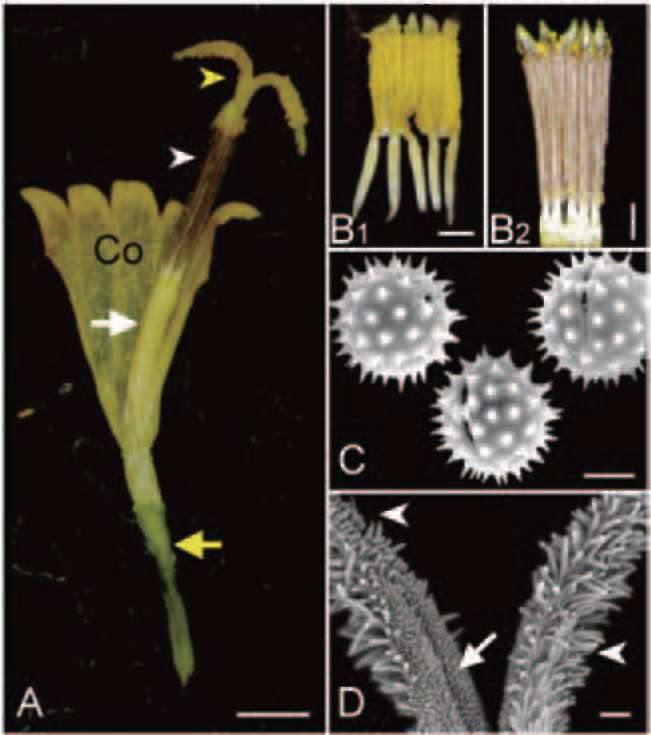
Figure 2. Disk floret and its reproductive components of Bidens pilosa var. radiata. (A) a dissected disk floret consisting five joined petals (forming a tubular corolla, Co), five stamens with united brown anthers (forming an anther tube, white arrowhead) and white filaments free from each other (white arrow), one style with two stylar arms (yellow arrowhead) situated in the center of the anther tube, and an inferior ovary (yellow arrow) (bar = 1 mm); (B1) a dissected anther tube full of pollen grains before anthesis (bar = 200 fim); (B2) a dissected anther tube with few pollen grains remained after the style branches growing out of the anther tube (bar = 200 fim); (C) the equatorial view (the right two) and the polar view (the left one) of the tricolporate pollen grains with echinate ornaments (bar = 10 fim); (D) the branches of style tip with sweeping hairs (or brushing hairs) on the tip and abaxial surface (white arrowhead) and stigmatic papillae on the adaxial surface (white arrow) (bar = 100 fim).
echinate globular with tricolporate. After the style grew out of the anther tube, only a few pollen grains would be left in the anther tube (Figure 2B2).
Style tip was covered by two kinds of microstructure (Figure 2D). The tip and abaxial surface of the style branches were covered by longer brushing hairs (or sweeping hairs) (Figure 2D) while the adaxial surface (the stigmatic area) was occupied with smaller and shorter papillae (Figure 2D).
Flowering process and floral stage
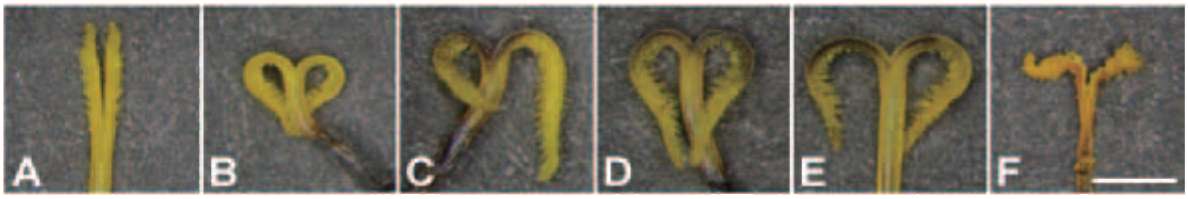
Flowering of B. pilosa var. radiata occurred continually in the field. A capitulum of B. pilosa var. radiata began anthesis with maturation of sterile ligulate florets. Following up, disk florets opened sequentially, about one whorl per day, from periphery to the centre of a capitulum. The flowering duration from the first disk floret to the final one was approximately four to six days.
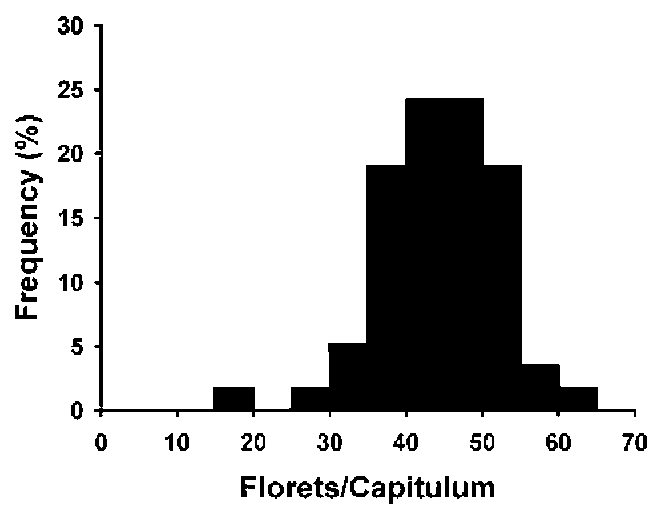
Figure 1. Normal frequency distribution (Kolmogorov-Smirnov test, D = 0.096, n = 58, p > 0.15) of hermaphroditic disk florets number in a capitulum of Bidens pilosa var. radiata.