HO et al. ― The genus Pythium in Hainan, China
527
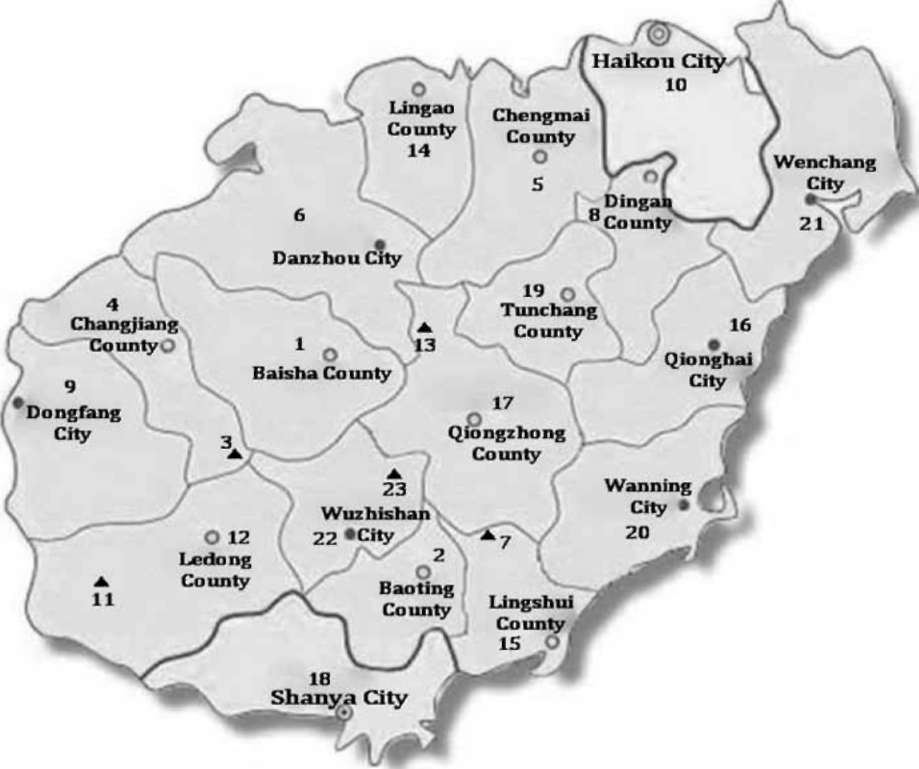
Figure 1. Distribution of Pythium species on Hainan Island. 1, Baisha County; 2, Baoting County; 3, Bawangling (Mountain); 4, Changji-ang City; 5, Chengmai County; 6, Danzhou City; 7, Diaoluoshan (Mountain); 8, Dongfang City; 9, Dingan County; 10, Haikao City; 11, Jianfengling (Mountain); 12, Ledong City; 13, Limushan (Mountain); 1 4, Lingao County; 1 5, Lingshui County; 16, Qionghai City; 17, Qion-gzhong County; 18, Shanya City; 19, Tungchang County; 20, Wanning City; 21, Wenchang City; 22, Wuzhis-han City; 23, Wuzhishan (Mountain).
after 12-24 hr at room temperature (ca. 25-28°C), surface cleaned with detergent, blotted dry and divided into small-er pieces (ca. 2x2 mm) which were then placed on 9-cm diam. selective agar media plates. There is no selective agar medium specifically for Pythium species only. The se-lective agar medium was formulated to isolate oomycetes: primarily Pythium and Phytophthora. It was prepared as follows: 3.5 g CaCo3was mixed thoroughly with 160 ml Campbell V-8 juice and the mixture clarified by filtra-tion through four layers of cheese cloth. Approximately 100 ml of clarified V-8 juice was diluted with distilled water to 1000 ml, boiled to dissolve 20 g Bacto agar and autoclaved at 121°C for 15 min. Prior to pouring the agar medium into sterilized Petri dishes, benomyl (200 μg /ml), pentachloronitrobenzene (100 μg /ml), rifampicin (100 μg /ml), ampicillin (200 fig^nl) and nystatin (50 μ/ml) were added. The inoculated plates were incubated at room temperature. When mycelial colonies appeared in 1-3 days, they were examined under an inverted light microscope to determine their identity as Pythium spp., which could be differentiated from Phytophthora spp. based on the much faster growth rate and the fine and flexuous hyphae (5-10 fim wide) (Erwin and Ribeiro, 1996). The hyphal tips were transferred to another selective agar plate before the isolate was finally cultured onto regular clarified V-8 juice agar medium. Pythium isolates were maintained on V8 agar slants and in small screw-capped glass vials (1.5x10 cm) of sterile distilled water.
Morphological studies
All morphological studies were made by growing the isolates onto 6-cm clarified V-8 juice agar plates. To in-
duce the production of sporangia, small pieces of mycelial agar discs (ca. 2x2 mm) were cut out from the edge of a growing colony and transferred to 6-cm or 9-cm Petri dishes with added sterile distilled water just enough to submerge the mycelial discs. They were left under regular indoor light at room temperature and checked daily with an inverted light microscope for sporangia, which usually appeared in 24-48 h. In most cases, sex organs appeared later in water or on agar plates. When the isolates failed to produce sporangia and/or sex organs in water cultures, small pieces of boiled grass leaves (ca. 2x2 mm) were added to stimulate their production. Alternatively, non-sterile stream/pond water or soil extract was used instead of sterile distilled water to flood the plates. In the case of P. splendens which is heterothallic, a pair of plus and minus strains were obtained through the courtesy of Dr. W.H. Ko of the University of Hawaii and individual isolates were paired with one of these mating strains on the same agar plate. They were examined under light microscope for the production of sex organs at the junction of both colonies after 2 wk. For detailed microscopic examinations, small agar discs bearing sporangia or sex organs were mounted directly in a drop of 0.05% cotton bluelactophenol on a glass slide and minced carefully into smaller pieces with a pair of l ml syringe needles. Then a 20x40 mm cover slip was placed on top with slight pressure to spread out the agar and the fungal structures. They were examined and photographed with a Nikon research light microscope (Japan Nikon Corporation, Tokyo, Japan) by interference contrast microscopy. Fifty measurements were made for each reproductive structure at a magnification of x400. Only normal, fully mature sporangia and sex organs were