HUANG et al. ― Trypsin inhibitor with thioltransferase-like and glutathione S-transferase-like activities
445
oxidation of NADPH were recorded. The TTase assay of HED reduction with GSH catalyzed by the SPTI sample solution was coupled to glutathione reductase and the consumption of NADPH was monitored at 340 nm. STI was used as a positive control. The disappearance of NADPH indicated by decrease at 340 nm was a measure of enzyme activity and extinction coefficient EmM (340 nm) = 6.22 were used for calculations.
Glutathione S-transferase assay
GSH conjugating activity was determined spectropho-tometrically as described by Singh and Shaw (1988) with a slight modification. Enzyme activity was assayed by combining 50 (l of 20 mM 1-chloro-2,4-dinitrobenzene
(CDNB), 50 (l of 20 mM reduced glutathione (GSH), 850
(l of 50 mM sodium phosphate buffer (pH 6.0 or 7.0), and 50 (l of an aliquot of the SPTI sample solution, then monitoring the change of absorbance at 340 nm (A340 nm), which is the UV absorption maximum of conjugating product between GSH and CDBN, at 25°C for 10 min. All initial rates were corrected for the background nonenzy-matic reaction. STI was used as a positive control.
Statistical Analysis
Means of triplicates were measured. Student's t test was used for comparison between two treatments. A difference was considered to be statistically significant whenp<0.05.
RESULTS
Effect of pH (7.5 and 8.5) on thioltransferase-like activity of SPTI
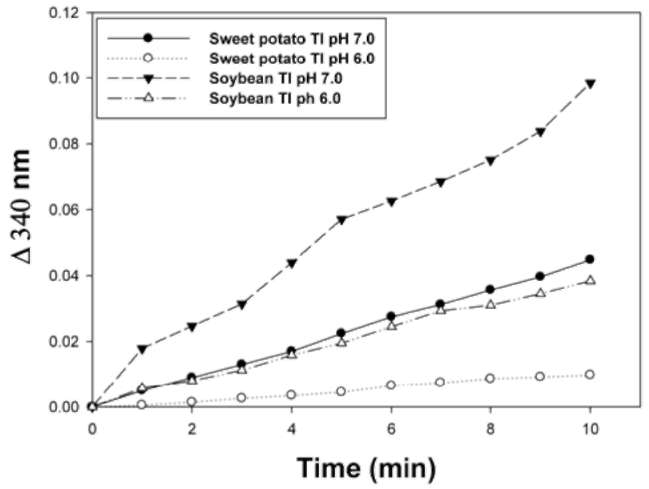
The assay using GSH and HED as substrates has been
used to identify TTase in plants (Morell et al., 1995). SPTI
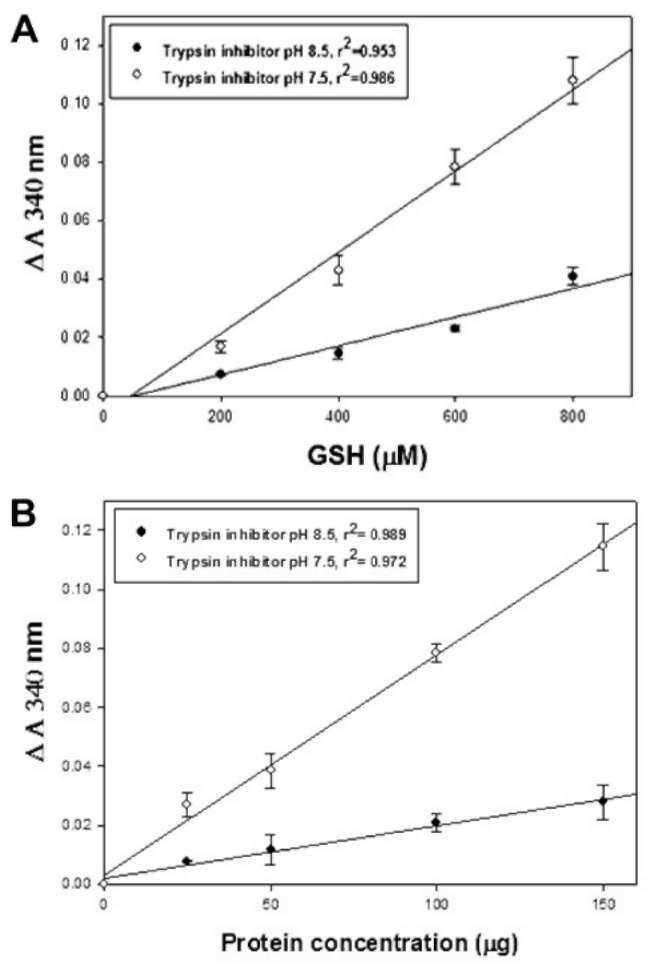
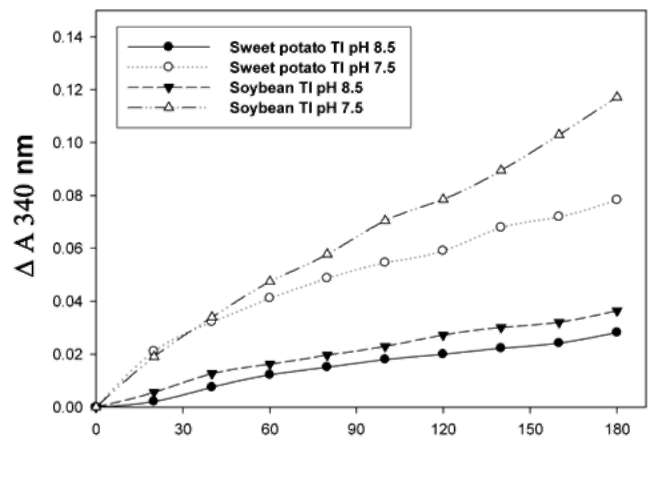
catalyzed the reduction of HED by glutathione; and the oxidized glutathione was then reduced back to its original form by the glutathione reductase using NADPH as a reducing equivalent in the coupled reaction (Figure 1). The specific TTase activity of SPTI was 1.6 士 0.3 and 0.58 士 0.02 nmol/min/mg protein at pH 7.5 and 8.5, respectively. STI was used as a positive control with specific activity of 2.4 士 0.5 and 0.75 士 0.03 nmol/min/mg protein at pH 7.5 and 8.5, respectively. Morell et al. reported a specific TTase activity of STI as 1.3 nmol/min/mg protein at pH 8.7 (Morell et al., 1995). Since they used crude extract of soybean, the real value might be higher than what they reported. Very low TTase-like activity of SPTI was found
at pH 8.5. SPTI exhibits a GSH-dependent TTase-like
activity (Figure 2A) at both pH 7.5 and 8.5. With coupled reaction, the rate of NADPH consumption is essentially proportional to SPTI concentration (Figure 2B). Thus, SPTI exhibits a GSH-dependent TTase-like activity at pH
7.5 and 8.5.
Effect of pH (6.0 and 7.0) on glutathione S-transferase-like activity
The GST activity of SPTI was determined with both
Time (sec)
Figure 1. Effect of pH (7.5 and 8.5) on thiotransferase-like activity of SPTI. The reaction mixture contained 50 mM Tris-HCl (pH 7.5 or 8.5), 2 mM EDTA, 300 (iM NADPH, 200 (iM GSH, 0.5 units glutathione reductase, and 100 (ig SPTI. The reaction was started by adding 450 (iL reaction mixture to a cuvette containing 50 (iL of 15 mM HED. Decreases of absorbance at 340 nm due to the oxidation of NADPH were recorded for 3 min. STI was used as a positive control. Each data show the mean 士 SD of one experiment performed in triplicate.
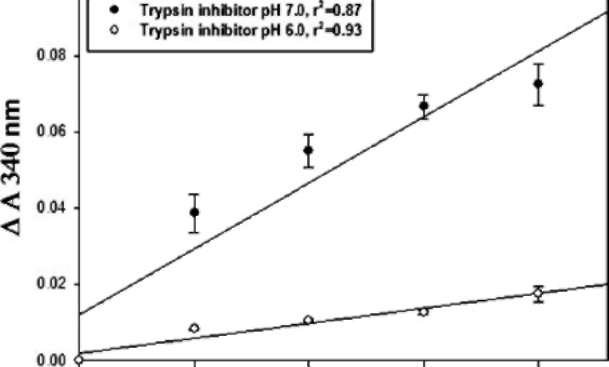
glutathione and CDNB as substrates. Figure 3 shows that
SPTI exhibited GST-like activity at both pH 6.0 and 7.0.
The specific GSTs activity of SPTI was 0.094 士 0.005 and 0.43 士 0.03 [imol/min/mg protein at 6.0 and 7.0, respectively. STI was used as a positive control with a specific GSTs activity of 0.37 ± 0.08 and 0.94 ± 0.06 [imol/min/mg protein at pH 6.0 and 7.0, respectively. However, very low GST-like activity of SPTI was found at pH 6.0. The rate of
GSH-dependent GST-like activities at both pH 6.0 and 7.0,
was dependant on concentrations of both GSH (Figure 4A)
and SPTI (Figure 4B).
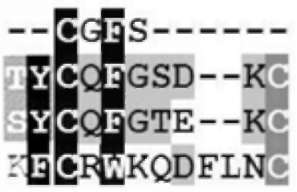
Comparison of amino acid sequences around disulfide bond of SPTI (sporamin A and B) and glutathione S-transferase, and the active site of thioltransferase
Amino acids around disulfide bond (Cys153~Cys160) of SPTI (sporamin A and sporamin B) were compared with those of sweet potato GST (CB330391) and the active site of TTase by CLUSTALX 1.81 software (Table 1). The comparisons provide some molecular clues of the origin of both glutathione S-transferase and thioltransferase activities of SPTI polypeptides here.
DISCUSSION
Although SPTI exhibit neither very high thioltransfer-ase-like or glutathione S-transferase-like specific activities, these activities may still be of physiological significance because the large amounts of SPTI in sweet potato storage roots or leaves (Lin and Chen, 1980) may compensate for the low specific activities.