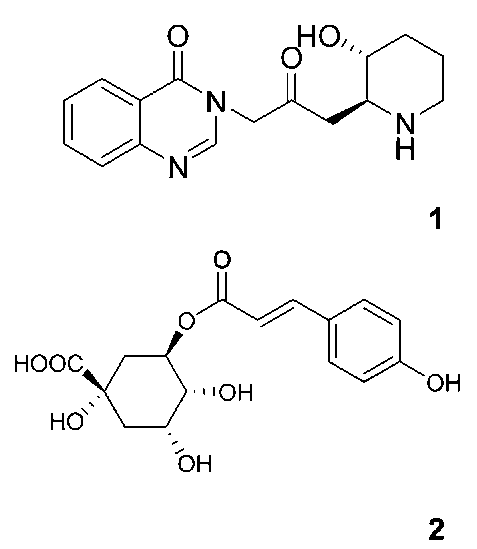
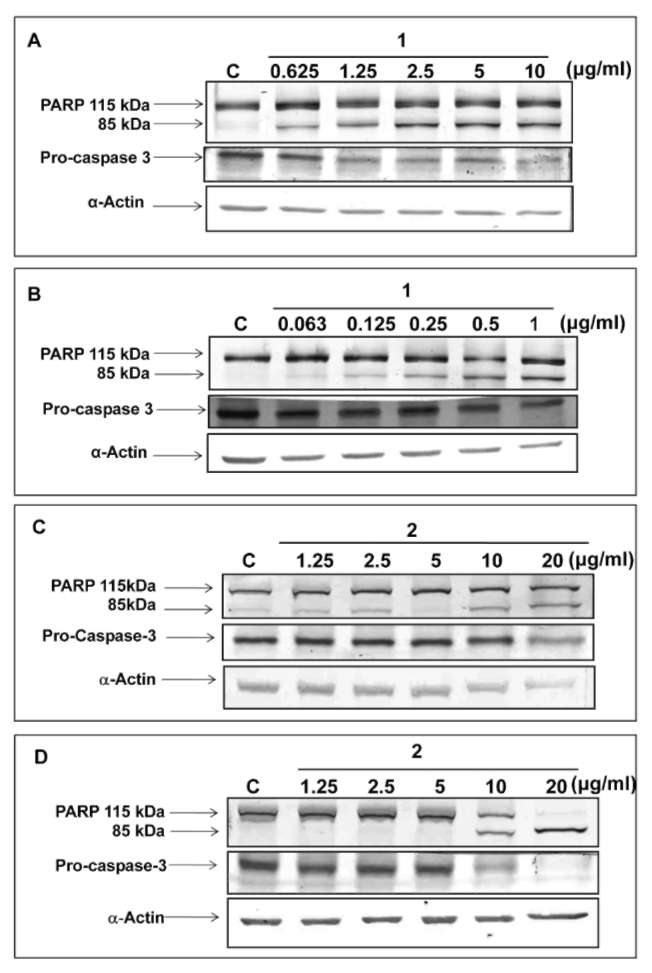
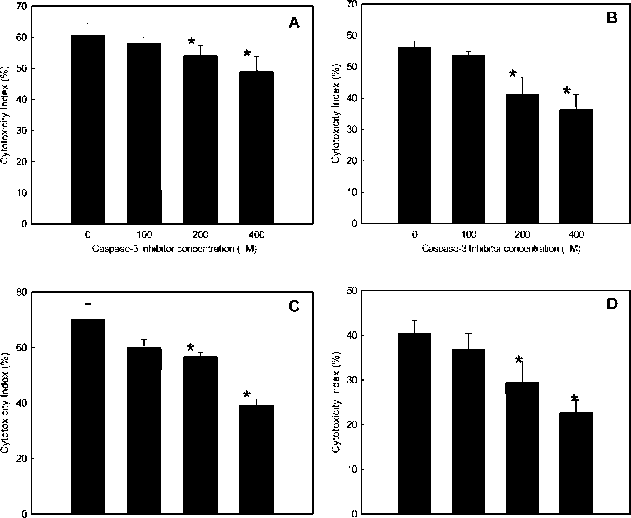
HSIEH et al. ― Cytotoxic constituents of Hydrangea angustipetala
47
and resolved by denaturing SDS-PAGE, using standard methods (Yang et al., 2003). The proteins were transferred onto an Immobilon-P PVDF 0. 45 -f m membrane
(IPVH00010, Millipore, USA), and Western blotting
was performed using antibodies specific to human PARP, pro-caspase, and a-actin. A goat anti-mouse antibody
conjugated to alkaline phosphatase and BCIP/NBT (BCIP/
NBT, Gibco) were used to visualize the protein bands.
RESULTS AND DISCUSSION
The leaves and flowers of H. angustipetala were extracted with 70% acetone after homogenization and the cytotoxic effects were measured by an MTT assay in AGS cells. The leaves of 70% acetone H. angustipetala extract (70A) exhibited the stronger cytotoxicity against AGS cells than the flowers (Table 1). The 70% acetone extract of its leaves (70A) was more cytotoxic than 50% EtOH extract in AGS cells. Furthermore, the 70A inhibited the growth of AGS and SNU-1 cells in dose- and time-dependent manners (Figure 1A, B, Table 2).
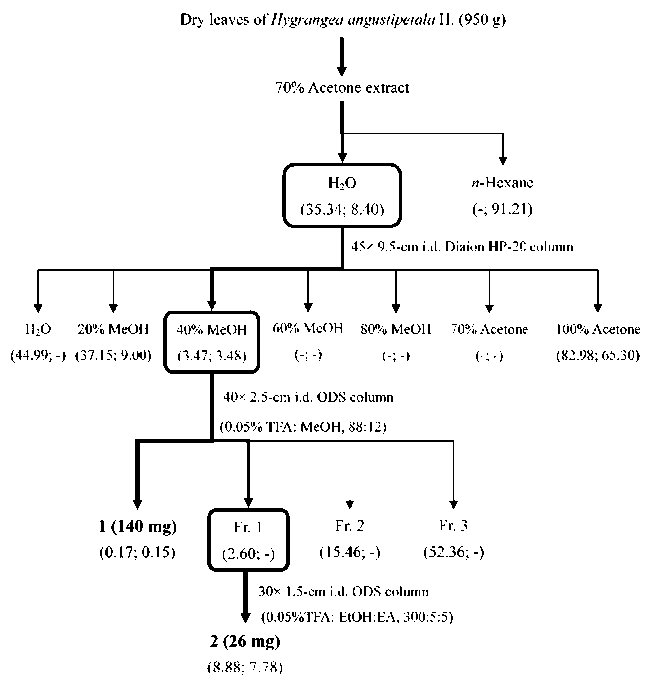
The chemical prescreening tests for H. angustipetala indicated that the flowers were richer in polyphenols than the leaves (Table 1) and suggested that the 70A contained phenol groups. Therefore, the 70% aqueous extract of H. angustipetala leaves was portioned with n-hexane to give n-hexane and aqueous (H2O) layers. Based on the cytotoxicity assay guide for the three fractionations, the H2O layer was more cytotoxic than the n-hexane one (Figure 2). The H2O layer was chromatographed on a
Diaion HP-20 column to give seven eluted fractions, and their cytotoxic effects in AGS and SNU-1 cells are shown in Figure 2. Of the seven fractions, fraction III (the 40% MeOH-eluted fraction, D40M) displayed strongest cytotoxic effect. Therefore, the antitumor effects of D40M were evaluated using P-388D1 cells in in vitro and in vivo models. As shown in Table 3, D40M strongly inhibited the growth of P-388D1 cells and the IC50 value was 0.72 fg/ml. Moreover, D40M at 10 mg/kg body weight significantly prolonged the life span of P-388D1 tumor-bearing CDF1 mice by 21.4% compared to the 1% DMSO solvent-treated mice. According to the above results, we suggested that
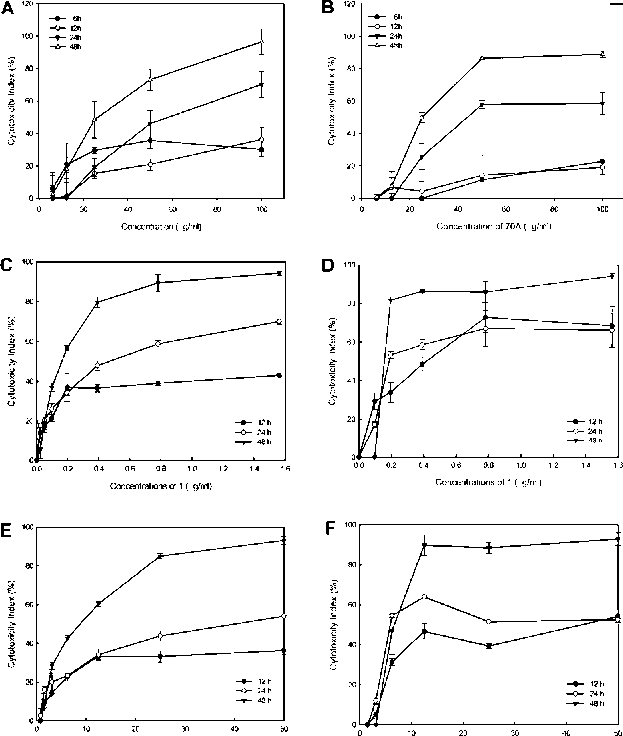
Table 1. Chemical and bioactivity prescreening tests of Hydrangea angustipetala leaves and flowers.
|
Extracted solvent Used part
|
70% Acetone
|
50% EtOH
|
|||
|
Flower
|
Leaf
|
Leaf
|
|||
|
10% NaOHa
|
+++
|
++
|
+
|
||
|
Fed; solution1"
|
+++
|
-
|
-
|
||
|
Cytotoxicity*1
|
49.31
|
21.43
|
31.08
|
||
Figure 1. Cytotoxic effects of tested samples used to treat AGS and SNU-1 cells in dose- and time-dependent manners. The 70% actone extract (70A)-treated AGS (A) and SNU-1 cells (B); (+)-febrifugine (1)-treated AGS (C) and SNU-1 cells (D); and trans-3-p-coumaroylquinic acid (2)-treated AGS (E) SNU-1
cells (F).
a+, the tested solution turned yellow. b+, the test solution turned blackish-green. cIC50 values (ig/ml) of extracts in AGS cells for 48 h. Data were used from three separate experiments.
Table 2. IC50 values of the 70% acetone extract (70A), (+)-febrifugine (1) and trans-3-p-coumaroylquinic acid (2) in gastric carcinoma cell lines (AGS, SNU-1).
|
|
|
|
|
IC50 (ig/ml)
|
|
|
|
||
|
|
|
|
AGS
|
|
|
SNU-1
|
|
||
|
Time (h)
|
70A
|
1
|
2
|
DOXa 70A
|
1
|
2
|
DOXa
|
||
|
12
|
-
|
5.20
|
76.97
|
--
|
0.43
|
40.52
|
-
|
||
|
24
|
71.49
|
0.43
|
40.84
|
- 43.87
|
0.19
|
5.98
|
0.04
|
||
|
48
|
21.43
|
0.17
|
8.88
|
0.06 30.18
|
0.05
|
7.78
|
-
|
||
aDOX was doxorubicin as a positive control. -, Not detected. Data were used from three separate experiments.