82
Botanical Studies, Vol. 51, 2010
floral morphs (Barrett and Forno, 1982; Ater, 2005; Castro
et al., 2007; Lou et al., 2006).
Oxalis corymbosa DC. (section Ionoxalis, Oxalidaceae), a species native to South America (Lourteig, 1980), was introduced into Taiwan as an ornamental plant (Wu et al., 2004). This species belongs to a tristylous genus with strong self-incompatibility and has been reported to have sexual reproduction and to produce capsules in its native environment (Barker, 1965; Lourteig, 1980). In Taiwan, O. corymbosa has never been observed to produce fruits, and it reproduces vegetatively by bulbils (Huang and Liu, 1993). The bulbils persist in the soil for many years and are easily dispersed by anthropogenic activities, namely by moving soils for cultivation (Wu et al., 1978). As in Taiwan, this species as been described as reproducing only asexually in other areas in which it has been introduced, like North America, Europe (Denton, 1973; Lourteig, 1980), China (Lou et al., 2006), and Malaysia (Veldkamp, 1971).
Several factors have been suggested for the lack of or low seed production in Oxalis, and these include unequal proportions or the absence of reciprocal floral
morphs (Barker, 1965; Denton, 1973; Castro et al., 2007),
geographical separation between reciprocal floral morphs (Lou et al., 2006), scarcity of pollinators (Marco and
Arroyo, 1998), ovule abortion (Guth and Weller, 1986),
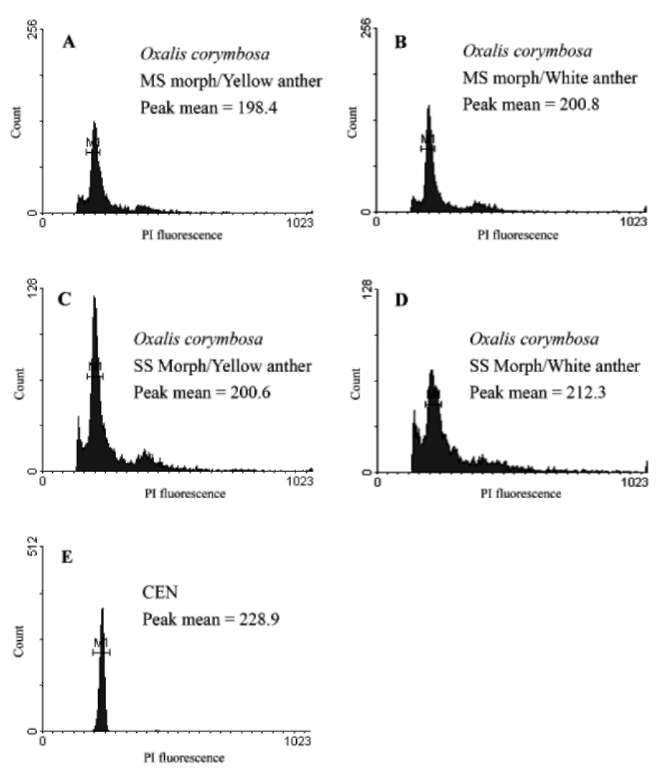
unviable pollen (Carniel, 1969), and meiotic problems due to polyploidy, odd ploidies, or aneuploidy (Baker, 1965; Castro et al., 2007). For example, in the Mediterranean region, populations of O. pes-caprae have long- and short-styled morphs, but the different floral morphs rarely grow in mixed populations and have different ploidies being unable to produce viable offspring (Castro et al., 2007). Previous studies developed in three populations of O. corymbosa in a close geographic range in Taiwan revealed that most of the populations were composed by the mid-styled morph (Wu et al., 1978). Additionally, O. corymbosa has also been reported with various chromosome numbers, 2n = 14 (Naranjo et al., 1982; Xu et al., 1992), 28 (Roy et al., 1988; Luo et al., 2006), 35 (Barker, 1965), corresponding to different ploidy levels, i.e., 2x, 4x and 5x, respectively. In light of these observations, it was our objective to understand the factors limiting sexual reproduction of O. corymbosa in the exotic range across Taiwan. For this, we expanded the survey and performed a large-scale study across Taiwan in order to (1) investigate the composition and distribution of floral morphs, (2) measure the pollen viability, and (3) assess the ploidy level of wild populations of O. corymbosa. Fruit and seed production were also assessed.
materials and methods
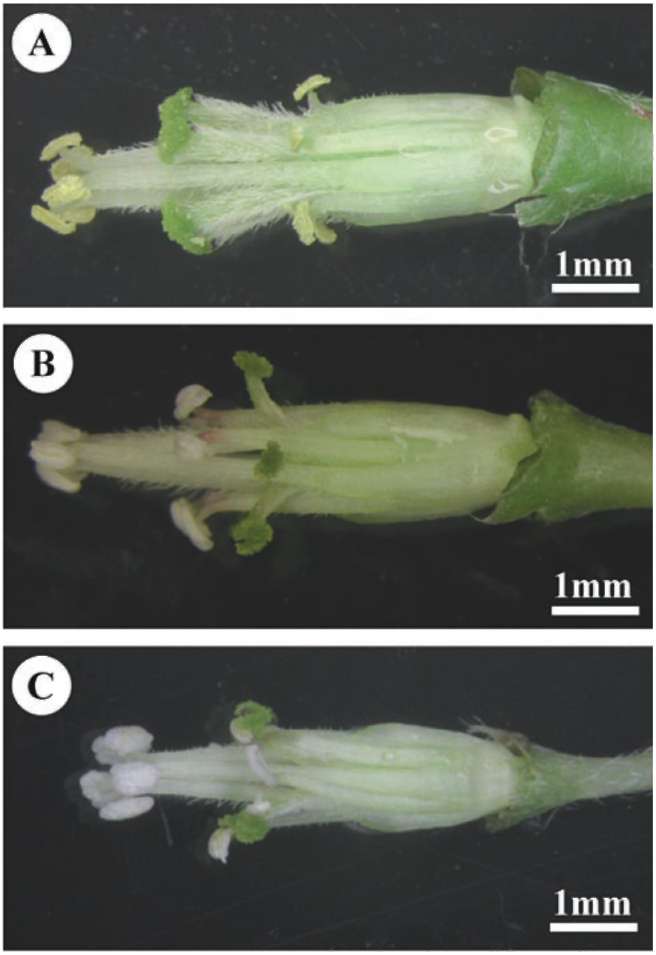
Oxalis corymbosa is a tristylic perennial herb with a tap-root and numerous bulbils. The flower is composed of five sepals, five petals (of a rose-purple color), ten statements in two rounds, and one round of five stigmas
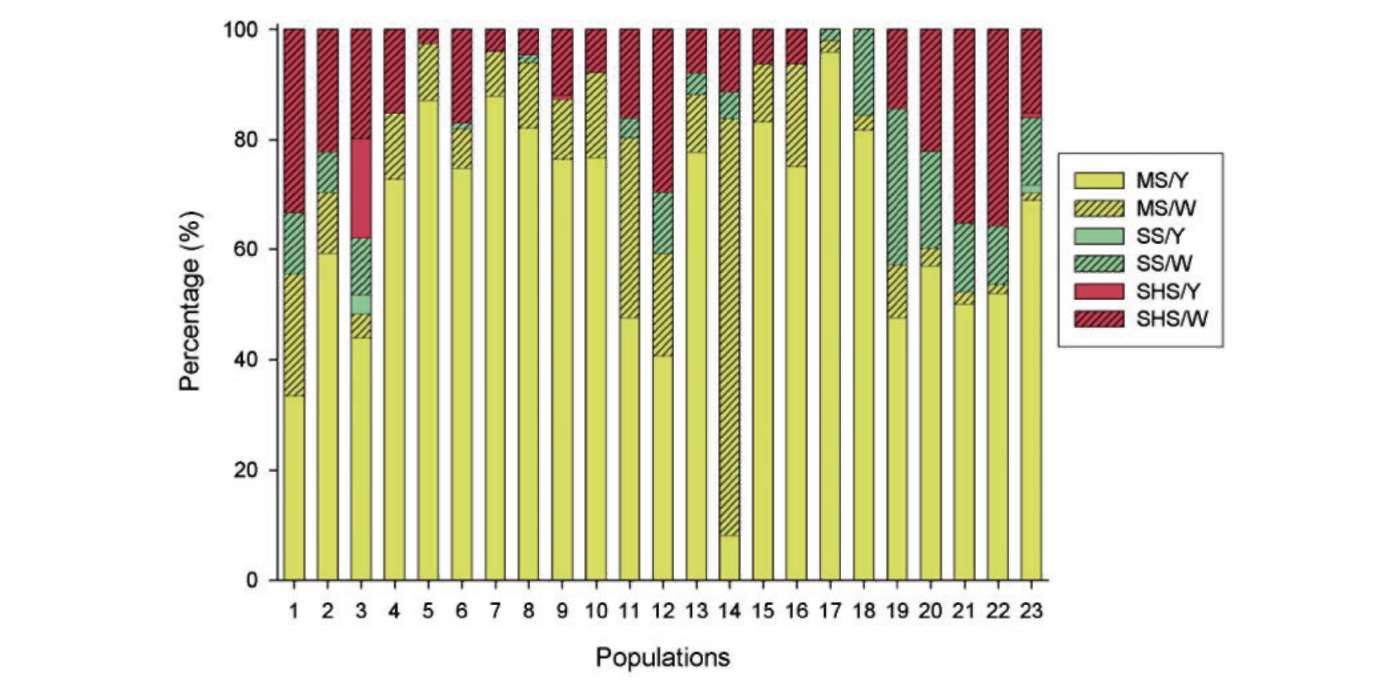
displayed in reciprocal heights (i.e., short-styled, SS, mid-styled, MS and long-styled morphs, LS; Figure 2). Furthermore, a semi-homostylous morph (SHS) with a long whorl of anthers and a short whorl of anthers and stigmas coinciding in height (Figure 2C) was also described in Taiwan (Wu et al. , 1 978). In Taiwan, it generally flowers in late winter to spring. Twenty-three natural populations of O. corymbosa in Taiwan were investigated between December 2005 and March 2007. Populations grow in disturbed places such as cultivated ground, roadsides, wasteland, parks and schools. We divided the administrative districts of Taiwan into four regions (northern, central, southern, and eastern regions), and all the populations were randomly selected including twelve populations (no. 1-12) from the northern region (Taipei, and Yilan), four populations (no. 13-16) from the central region (Chiayi), three populations (no. 17-19) from the southern region (Tainan, Kaohsiung, and Pintung), and four populations (no. 20-23) from the eastern region (Hualien, and Taitung) (Figure 1).
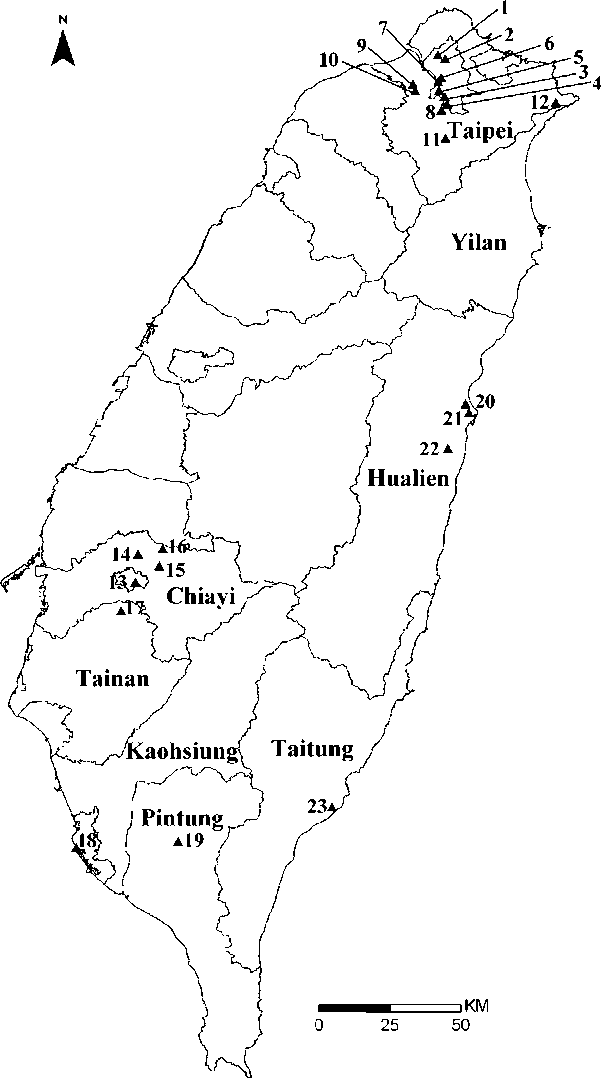
Figure 1. Map of Taiwan showing location of 23 populations of O. corymbosa investigated in this study.