156
Botanical Studies, Vol. 51, 2010
MATERIALS AND METHODS
Plant material and treatments
Seeds of P. tricuspidata were germinated in pots containing sand and peat (v/v 3:1). After 20 days, each seedling was transplanted into each plastic pot (30 cm high, 25 cm diameter) containing 5 kg clayed loam soil (pH 6.5, organic matter 22.08 g-kg-1, total N 1450 mg-kg-1, total P 1110 mg-kg-1, total K 780 mg-kg-1). At the same time, all the pots received an application of 0.6 g-kg-1 soil of fertilizer (N-P2O5-K2O, 15-15-15). Five levels of water supply treatments were imposed at the six-leaf seedling stage with field capacity at 5% (W1), 15% (W2), 30% (W3), 60% (W4), and 100% (W5). Each treatment was replicated 10 times. Water was applied by pouring it into three plastic pipes (diameter: 1 cm) placed in the soil in order to limit the heterogeneity of the soil water content (Flenet et al., 1996). To minimize soil evaporation, the soil surface was covered with plastic film. Throughout the experiment, pots were weighed daily at 16:00 to replenish lost water. The experiment was conducted in a greenhouse where the temperature was maintained at 30-35°C during the day and 20-25°C at night. Air humidity was 65%-70%.
Data collection and analysis
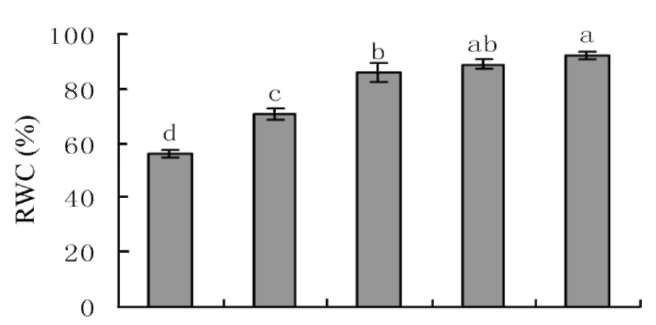
The leaf relative water content (RWC) was estimated by determining fresh mass, dry mass, and the mass at full turgor after hydrated for 24 h with distilled water (Kumar and Singh, 1998) and calculated as follows:
Wl W2 W3 W4 W5
Figure 1. Leaf relative water content (RWC) of P. tricuspidata seedling under different water supplies condition. Different letters in the column represent significant difference at P0 05 level by Duncan's new multiple range test.
software. The Duncan test was used to perform multiple comparisons at a significance level of P<0.05.
RESULTS
Leaf relative water content (RWC)
Leaf RWC reflects the water content in plant tissue and is often used as a parameter to assess the severity of water stress. Figure 1 showed that Leaf RWC significantly decreased along with the decreasing water supply which was 92% in W5, 89% in W4, 86% in W3, 71% in W2 and only 56% in W1, that reflected the sensitive response of P. tricuspidata for changing of soil water content.
Growth morphology
The morphology of P. tricuspidata seedlings was significantly affected by water supply (Table 1). Increasing water supply led to increase in stem diameter, internode length and petiole length of seedlings, but decrease root length. The branch number, total branch length, leaf number and total leaf area of seedlings grown under lower water supply was lower as compared to those seedlings grown under higher water supply. W4 had the highest stem length (79.7 cm) than other treatments. This result represented 68% decrease in W1 and 36% in W5 as compared with seedlings under W4 treatment.
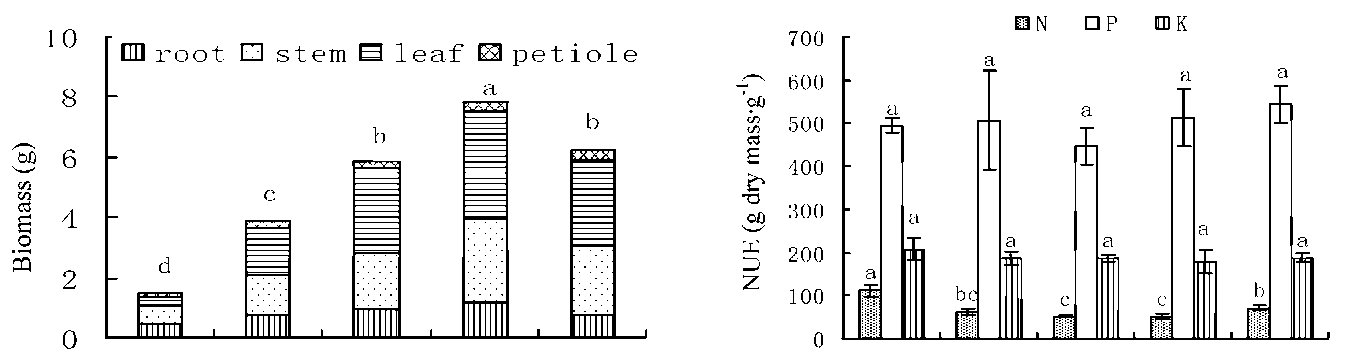
Dry biomass and biomass partition
Figure 2 showed that total biomass accumulation was significantly decreased with soil water supply decreasing from W4 to W1. Under lower soil water supplies, root growth constituted the largest proportion of the biomass (Figure 3). The ratio of root / above ground dry biomass partition had decreased along with increasing water supply. The highest value (0.44) was observed in W1 and the lowest value (0.15) in W5. The ratio of leaf / stem biomass under different water supply exceeded 1, except for W1 (0.49) (Figure 3). Under soil water supply between W2 and W5, leaf has accounted for more than 40% of total plant biomass, while stem, root and petiole accounted for less than 36%, 20% and 6%, respectively (Figure 3).
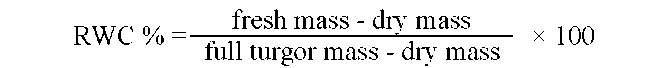
Seedlings were harvested 60 days after the fist induction of water supply treatments (from August to October). The measured morphological features measured were root length, stem length, stem diameter, internode length, petiole length, total shoot length, branch number and leaf number. The total leaf area per plant was measured using a Li-3000 leaf area meter (Li-COR, Lincoln, Nebraska). Each plant organs (leaves, stems, and roots) was dried at 70°C for 48 h. After dried completely, plant organs were ground and passed through a 20 mesh screen for the tissue nutrient (N, P and K) concentrations estimation (Ru, 2000). Total nutrient content of each tissue type was calculated by multiplying their respective dry mass by their tissue nutrient concentrations. Total plant dry mass or nutrient content was calculated as a sum of the dry mass or nutrient contents of an individual's stem, roots, and leaves.
Nutrient use efficiency (NUE) of N, P and K were calculated using the following equation (Baligar et al.,
2001):
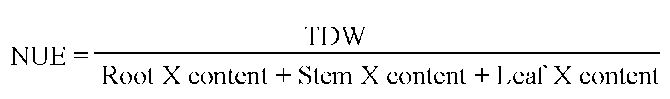
Where TDW is total dry weight of plant in g-plant-1; X stands for N, P or K content in g-plant-1.
Data were analyzed with STATISTICA package 5.0