164
Botanical Studies, Vol. 51, 2010
Taiwan (Liao, 1976). The fragrant sawdust of this species has long been used for joss sticks and thus it was named Taiwan incense cedar. Taiwan incense cedar is distributed in hillsides of natural forests between 300 and 1,800 m in elevations (Lu et al., 1994). However, the annual radial growth is slower compared to two other important sister species, Taiwan yellow cypress (Chamaecyparis obtusa var. formosana) and Taiwan red cypress (C. formosensis)
(Wang et al., 1987).
Most previous studies of compression wood formation focused on fast-growing tree species in the growing season. The purpose of the present study was to examine the role of IAA in compression wood formation in seedlings of Taiwan incense cedar during a growth pause period by monitoring the growth of cambium induced by IAA. Meanwhile, we also placed seedlings horizontally to investigate stimulation of compression wood formation with and without the presence of IAA. Based on cambial activity represented by cambium cell and differentiating xylem cell numbers as described by Timell (1 986), cambium growth activities were carefully examined among the different test conditions. We hypothesized that the compression wood would form during the mid-season growth pause, especially in a tardy growing tree, and the compression wood formation should exist against the stress caused by leaning or application of IAA, or both.
MATERIALS AND METHODS
Seasonal changes in cambial activity
Seasonal changes in cambium cell activity in a mature tree of Taiwan incense cedar were measured. The tree was on the campus of National Taiwan University and its D.B.H. (diameter at breast height) was 110 cm and height is about 12 m. Samples were collected from the upper sides of branches on 15 March, 9 April, 7 May, 7 June, 5 July, 8 August, 9 October, and 6 November 2004.
Seedling selection
Thirty-nine 2-year-old seedlings of Taiwan incense cedar (Calocedrus macrolepis var. formosana) from the nursery of Chiayi Forest District Office of Taiwan Forestry Bureau in south western Taiwan (23°28' N, 120°26' E) were transplanted into a greenhouse at the Department of Forestry and Resource Conservation, National Taiwan University, Taipei (25°00' N, 121°27' E) on 5 May 2005. After a 1-month acclimation, the average height of seedlings was 106.9 ± 13.3 (± SE) cm and average diameter of the trunks at 20 cm height was 7.45 ± 0.96 (± SE)
Seedling treatments
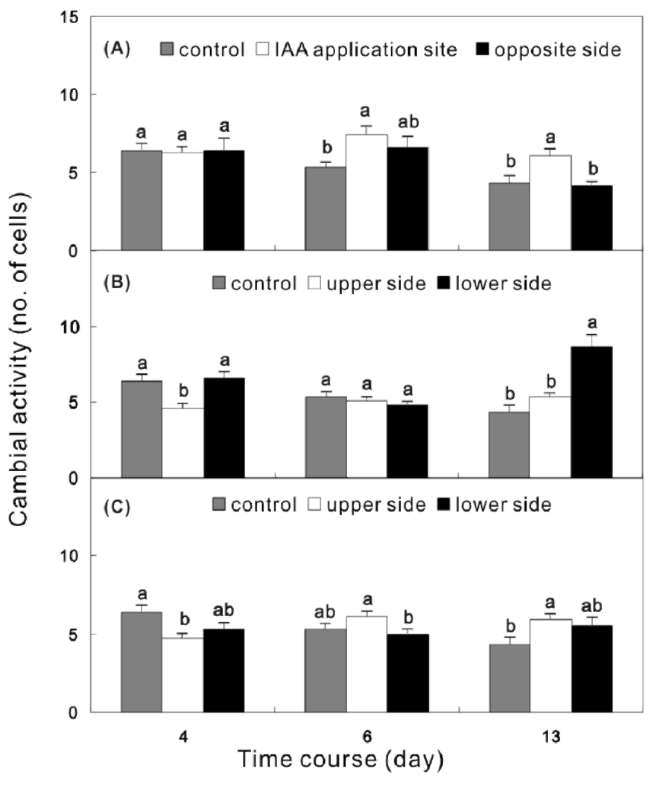
Seedlings were subdivided into four groups. Group 1 seedlings were planted vertically as the control (Figure 1A). Group 2 seedlings were planted vertically and marked at 25 cm above the basal part for cambium cell investigation (Figure 1B). For IAA treatment, the outer
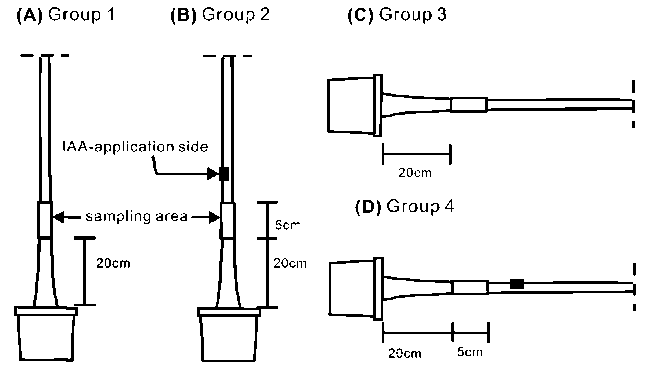
Figure 1. Experimental design. (A) Group 1, seedlings were kept standing as the control. (B) Group 2, seedlings were treated with IAA on one side. (C) Group 3, seedlings were leaned horizontally. (D) Group 4, seedlings were treated with IAA on the upper side of the stem and were leaned horizontally. The IAA application site was carefully oriented to be on the upper side of the trunk. Samples were collected 5 cm below the treated site. Each group contained three seedlings.
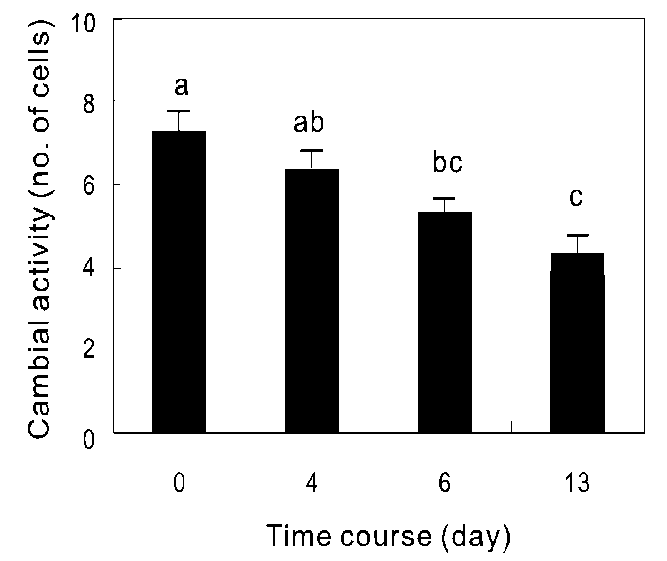
bark with a little secondary phloem tissue was cut using a knife blade to form a tangential wound (0.2 cm in length x 1 cm in width), and then 1% IAA-lanolin (80 |iL 5% IAA mixed with 400 mg melted lanolin) was directly applied to the wound site. The wound site was covered with aluminum foil and ventilated tape to protect the IAA from light (Figure 1B). Group 3 seedlings were laid horizontally to induce compression wood formation (Figure 1C). Group 4 seedlings were treated as group 2, but laid horizontally immediately after IAA application. In order to distinguish the compression wood induced by gravity, the IAA application site was carefully oriented so that it was on the upper side of the trunk (Figure 1D). The control group seedlings were harvested at 4 different times, i.e. days 0 (11 June 2005), 4 (15 June), 6 (17 June), and 13 (24 June), and three seedlings were harvested each time. The treatment groups were harvested after days 4, 6, and 13. Discs (1 cm in thickness) were collected from 20 cm above the basal part or 5 cm below the IAA application site of each seedling.
Cambium activity measurement
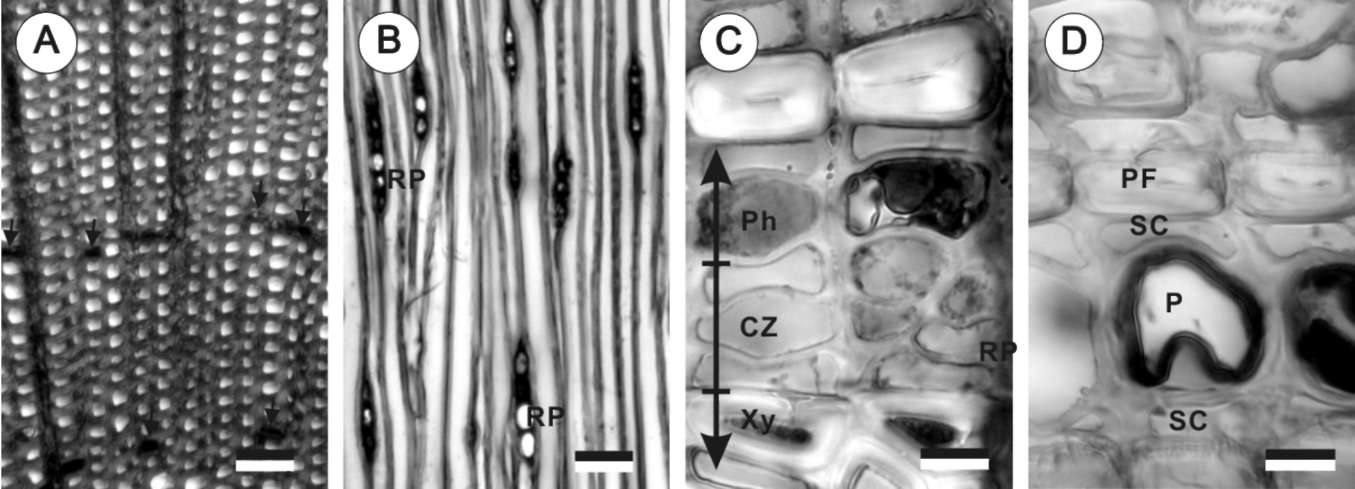
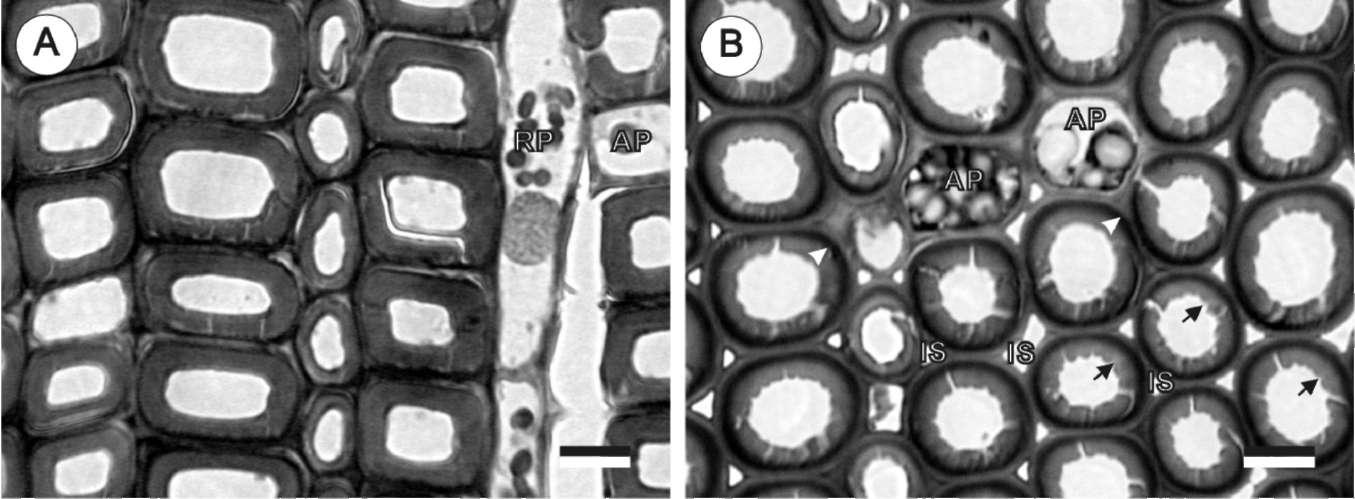
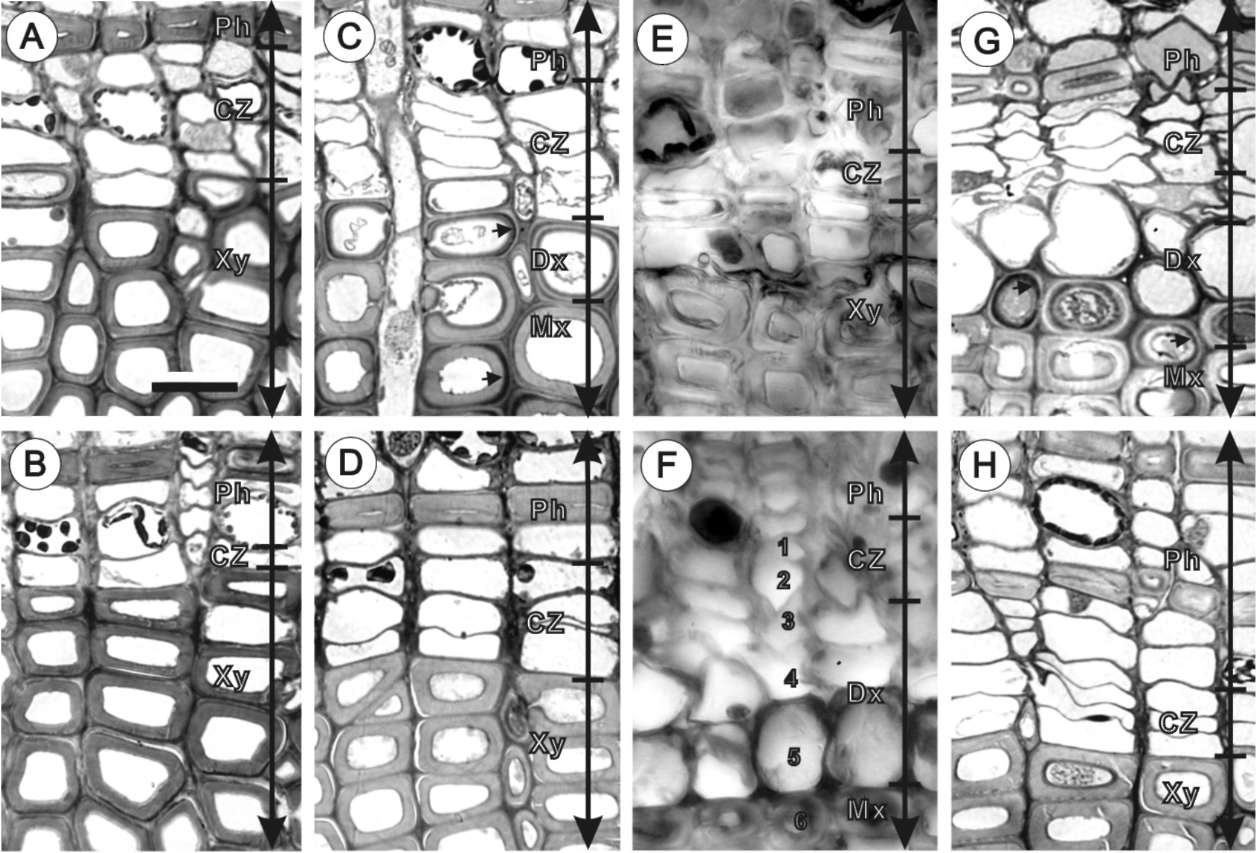
The cambium activity was measured by counting the numbers of vascular cambium and differentiating xylem cells in a single radial profile. Freshly cut discs (1 cm in thickness) were divided into two halves and immediately fixed in 1% glutaraldehyde (GA) in 0.1% sodium phosphate buffer (pH 7.0). Free hand sections from each disc were double-stained with 1% safranin O in a 50% ethanol solution and 1% fast green in a 95% ethanol solution. Under a light microscope (Leica Diaplan Microscope), cambium cells were counted. The counted cells included undifferentiated parenchymatous cells and differentiated xylem cells between the mature xylem and phloem. The cytoplasm of mature tracheids has already been degraded.