174
Botanical Studies, Vol. 51, 2010
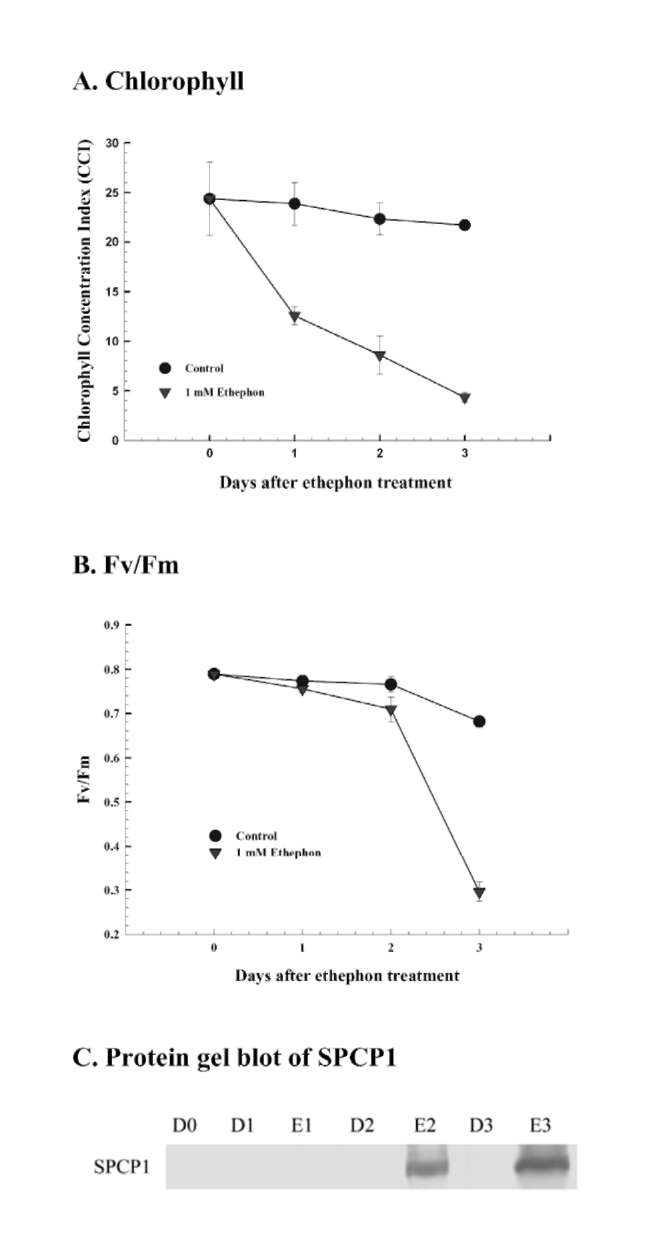
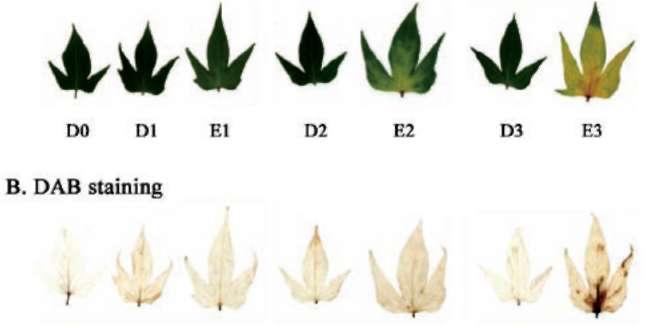
1A). Significant increase of H2O2 amount was observed at day 3 in ethephon treatment compared to dark control (Figure 1B). The chlorophyll content of detached leaves drastically decreased in ethephon treatment, and was about 17% that of D0 control at day 3. However, the chlorophyll content of dark control was not significantly varied and was about 89% that of D0 at day 3 (Figure 2A). The Fv/Fm value was also remarkably less and was about 38% that of D0 at day 3 in ethephon treatment. However, the Fv/Fm value of dark control was not significantly varied and was about 86% that of D0 control (Figure 2B). Cysteine protease SPCP1 expression was significantly enhanced from day 2 in ethephon treatment compared to untreated dark control (Figure 2C). These results clearly demonstrate that ethephon treatment can elevate H2O2 amount, reduce chlorophyll and Fv/Fm contents, induce cysteine protease SPCP1 expression, and promote leaf senescence in sweet potato detached leaves.
Ethephon-mediated effects were repressed by reduced glutathione
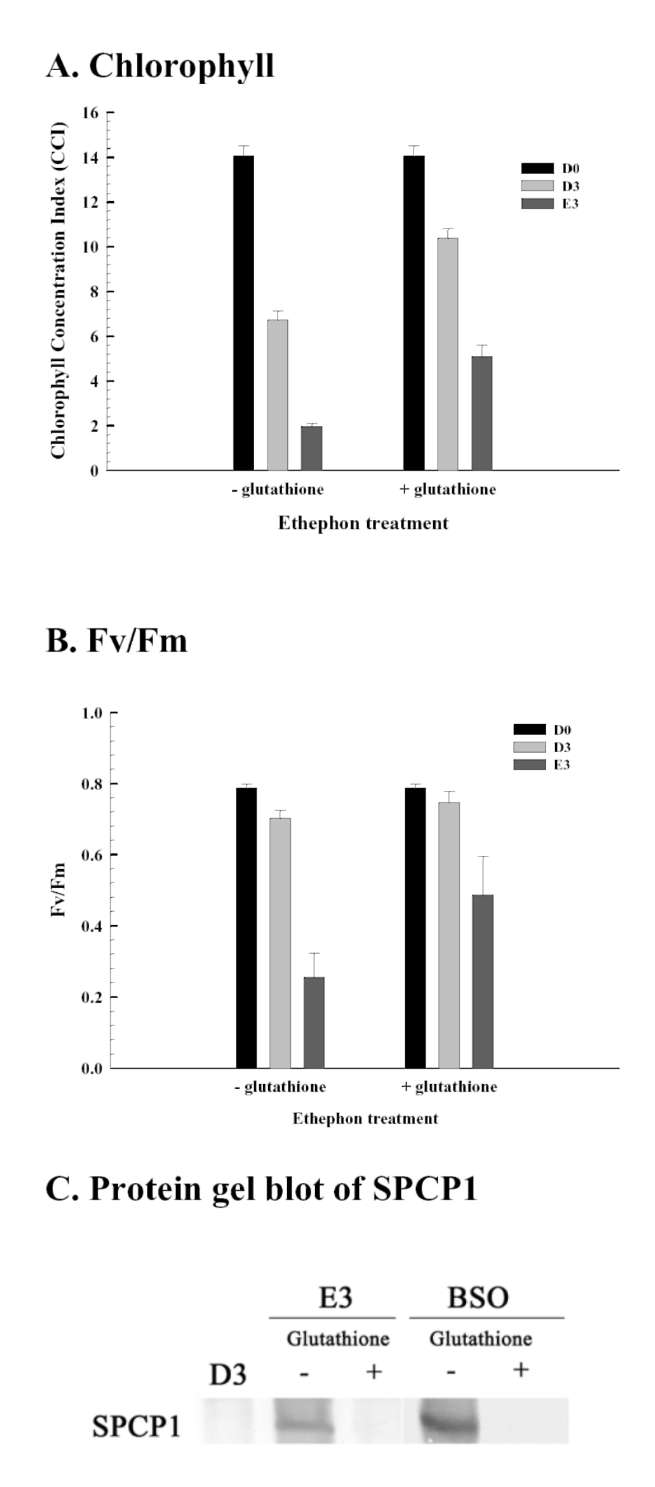
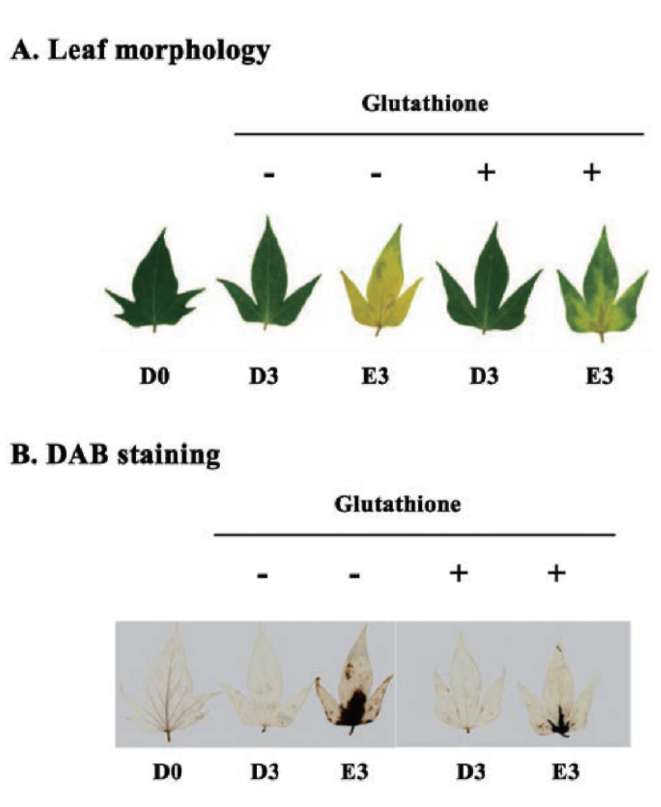
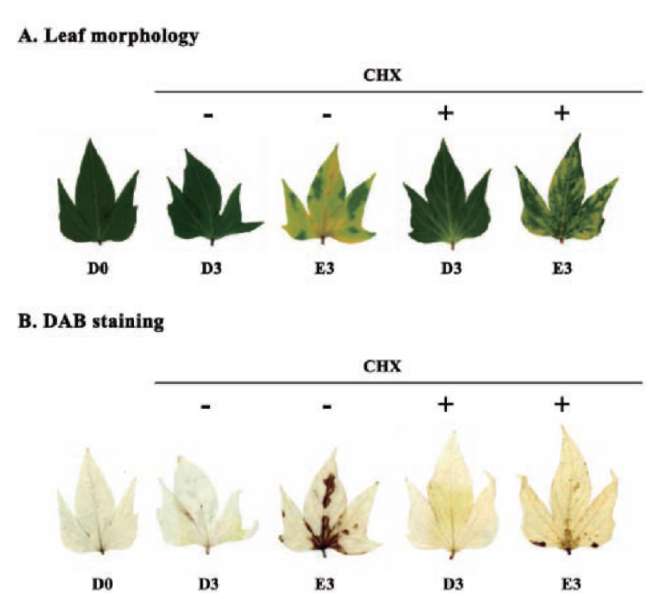
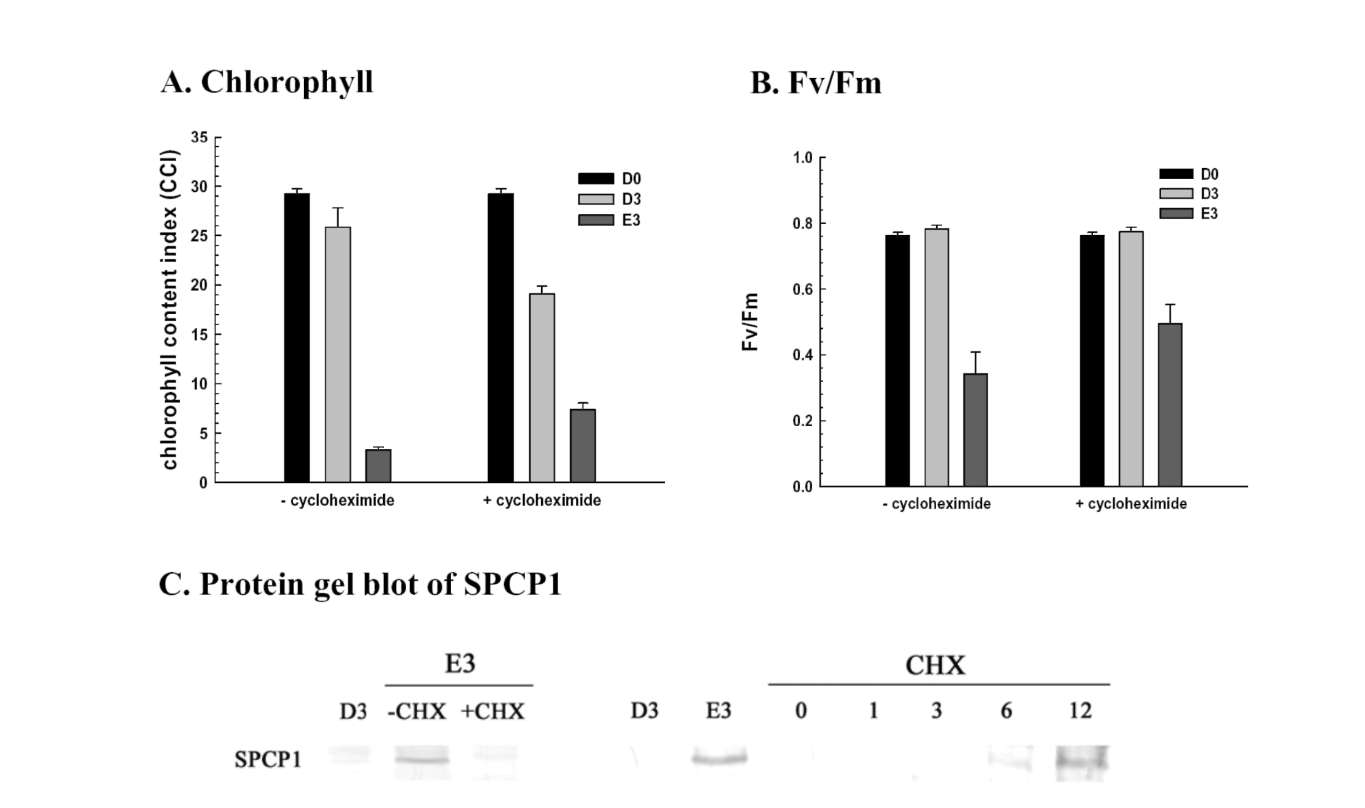
Reduced glutathione influence on ethephon-mediated induction of leaf senescence was studied. Sweet potato detached leaves senesced much earlier and almost turned yellow in ethephon treatment compared to dark control. However, ethephon-mediated effects were alleviated by reduced glutathione pretreatment. The degree of leaf senescence and H2O2 production at day 3 were drastically less in reduced glutathione pretreatment (Figure 3). The chlorophyll content of detached leaves at day 3 was about 14% and 36% that of D0 control for ethephon and ethephon plus reduced glutathione, respectively (Figure 4A). The Fv/Fm value also significantly decreased in ethephon treatment and was about 32% that of D0 control at day 3. However, reduced glutathione delayed the ethephon-mediated Fv/Fm reduction and was about 62% that of D0 control at day 3 (Figure 4B). Cysteine protease
Ai Leaf morphology
SPCP1 expression was enhanced in ethephon treatment compared to untreated dark control, and the induction at day 3 was repressed by reduced glutathione pretreatment (Figure 4C). These results clearly demonstrate that ethephon-mediated effects were significantly repressed by reduced glutathione pretreatment, and suggest that intracellular glutathione content may be important and involved in ethephon-mediated effects on leaf senescence and gene expression. Therefore, L-buthionine sulfoximide, which functions as an endogenous glutathione biosynthesis inhibitor, was used to induce SPCP1 expression, and the
DO Dl El D2 E2 D3 E3
Figure 1. Effects of ethephon on leaf senescence and oxidative stress. (A) Leaf morphology; (B) H2O2 detection with DAB staining in detached sweet potato leaves. Detached leaves were treated with 1 mM ethephon for 0, 1, 2 and 3 days, respectively. D and E denote dark and ethephon treatments, respectively. The experiments were performed three times and a representative one was shown.
Figure 2. Effects of ethephon on chlorophyll content, Fv/Fm
value, and cysteine protease SPCP1 expression in detached sweet potato leaves. (A) Chlorophyll content; (B) Fv/Fm value; (C) Protein gel blot of SPCP1 expression. Detached leaves were treated with 1 mM ethephon for 0, 1, 2 and 3 days, respectively. D and E denote dark and ethephon treatments, respectively. Protein gel blot was performed with polyclonal antibody raised previously against putative SPCP1 protein. The experiments were performed three times and a representative one was shown.