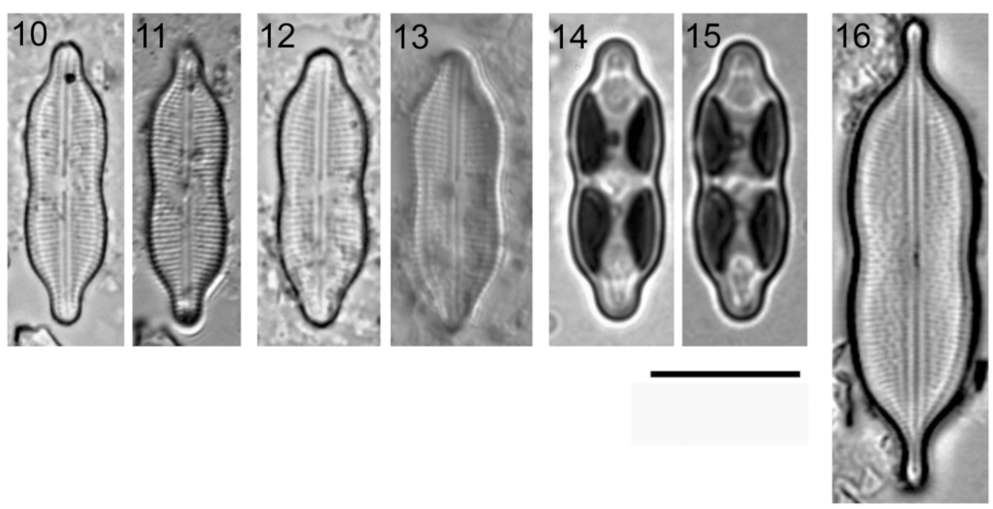
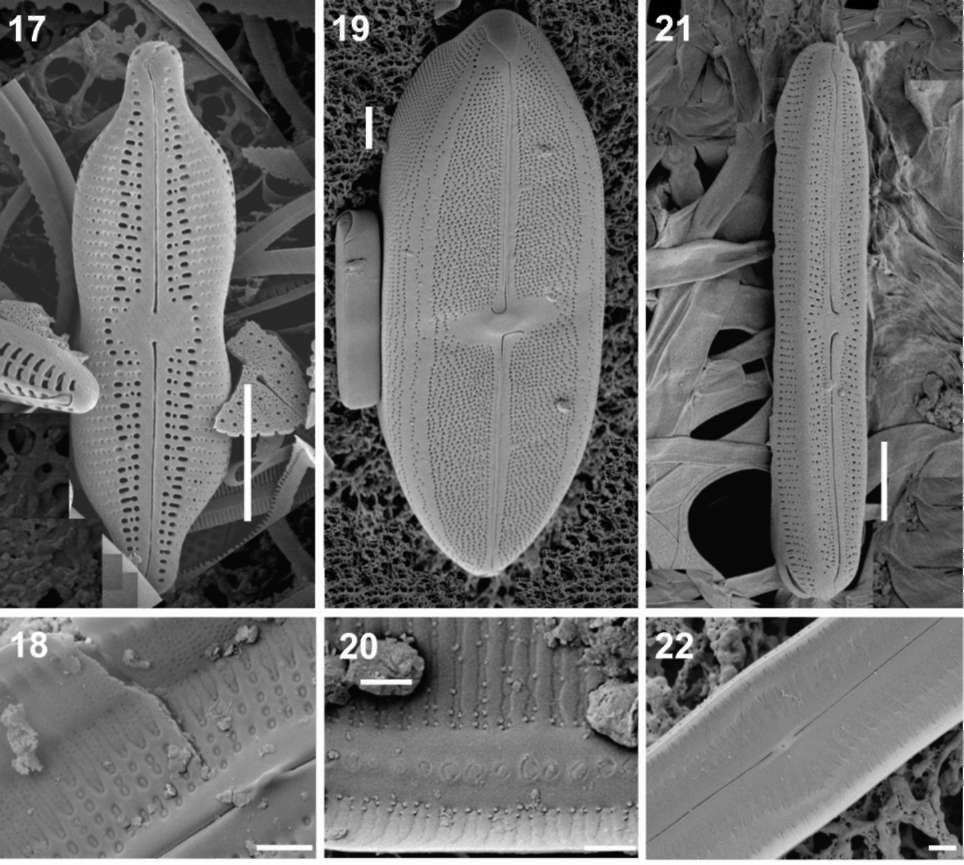
CANTONATI et al. ― Neidiomorpha gen. nov.
197
Striae transapicales abrupte transientes in seriebus fo-raminum minorum circularium densius sitorum inter se interruptae ad limbum foraminibus singulis amplis perval-variter elongatis. Lineae apicales hyalinae super canales longitudinales absunt.
Interne striae transapicales (sub-)marginales usque trans limbos pluriseriatae, hic membrana duplex cum caverna inclusa efficiens.
Cingulum paucis copulis apertis cum poris uniseriatis vel biseriatis constans.
Diagnosis. Naviculoid cells resembling Neidium with two plastids (until now observed only in the generitype species), appressed to one valve and to the girdle, arranged fore and aft, one in each half of the cell (Figures 14-15). The two so far precisely known species live in freshwater. Valves are linear with margins strongly con-
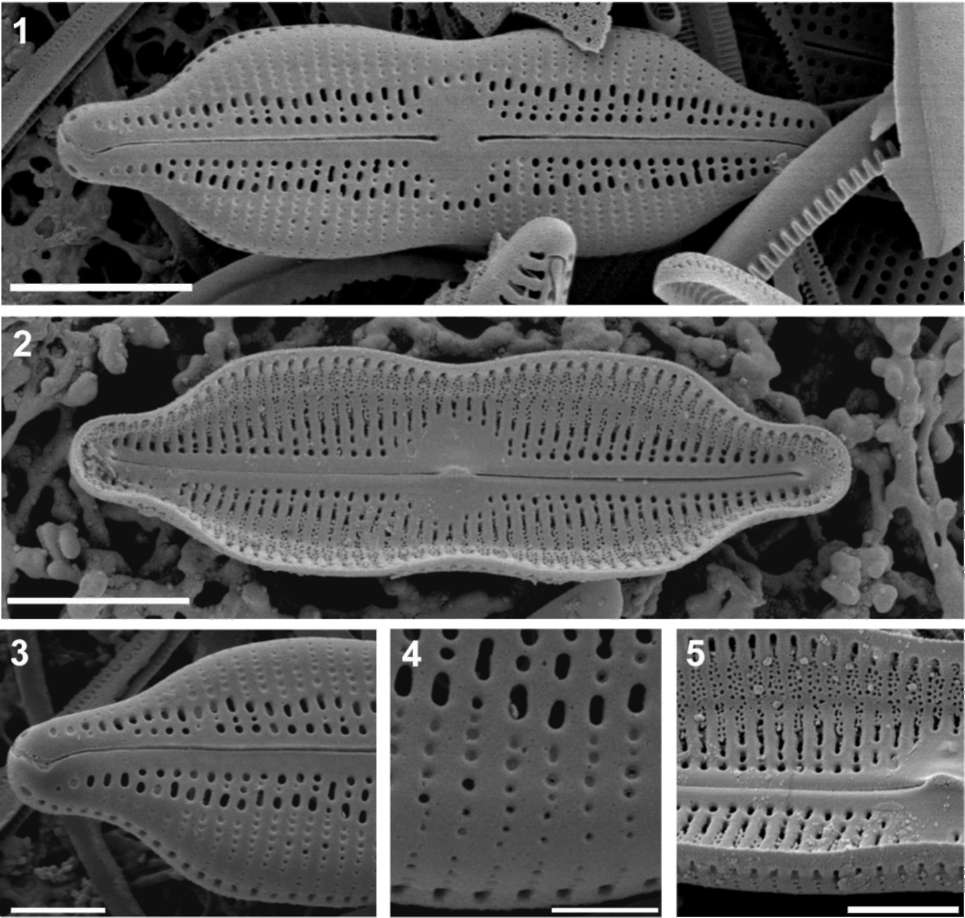
Figures 1-5. SEM micrographs of the new diatom genus (Neidiomorpha binodiformis nov. comb.). 1, Valve outside view (note external raphe structure and areolation pattern); 2, Inside view of the valve (note internal raphe structure and areolation pattern); 3, Polar part of the valve. Note the terminal raphe fissure, and the uniseriate areolae foramina forming 1-3 larger unoc-cluded apertures near the sternum becoming abruptly and distinctly smaller towards and onto the valve mantle; 4, Detailed outside view of the junction between valve face (upper part) and mantle (lower part). Note the outmost distal apertures of each stria forming a circumferential series of large areolae on the mantle; 5, Detailed inside view showing the proximal raphe end, the helictoglos-sa-like silica accumulation in correspondence of the central nodule, and inside pluriseriate areolation pattern over the shallow cave in the mantle that is consistently lacking in Neidium sensu stricto. Scale bars: Figures 1-2 = 5 (im, Figures 3, 5 = 2 (im, Figure 4 = 1 (im.
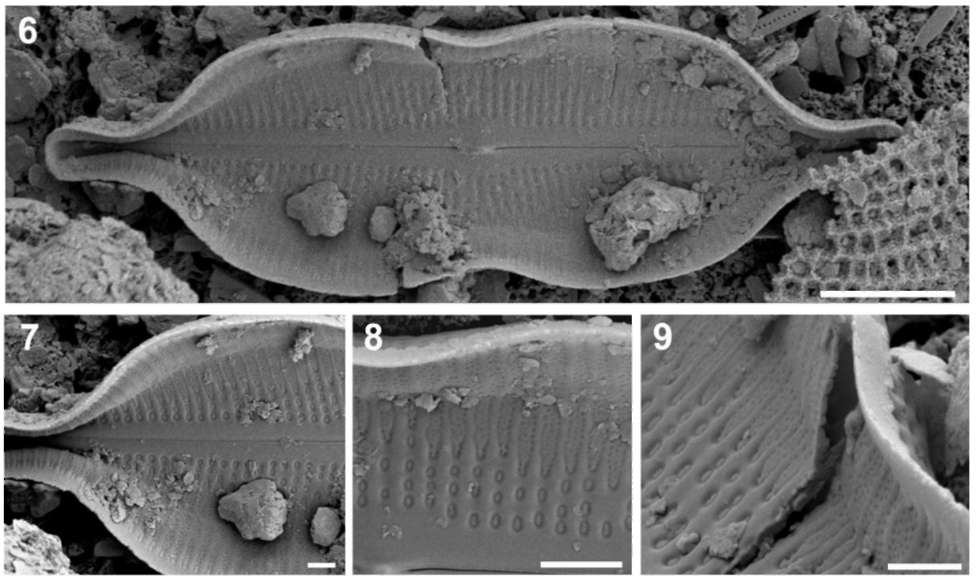
Figures 6-9. SEM micrographs, all inside view, of the new diatom genus (Neidiomorpha binodis nov. comb.; Hustedt material). 6, Entire valve (note internal raphe structure and central nodule); 7, Detail of the distal part (note inside areolation pattern); 8, Detail view on valve face and mantle, which appears inflated (note hymenes closing the areolae); 9, Detailed view showing a break in the marginal part of the valve that makes the structure of the shallow cave in the mantle visible. Scale bars: Figures 6 = 5 (im, Figures 7-9 =1 Lim.