210
Botanical Studies, Vol. 51, 2010
materials and methods
We collected seven Pteris cadieri (Figure 1A-G) and two P. grevilleana (Figure 1H-I) individuals with different frond morphology from five locations in Taiwan (Table 1). The living ferns were cultivated in the Taipei Botanical Garden greenhouse to examine their morphological variation. Voucher specimens were deposited in the Taiwan Forestry Research Institute herbarium (TAIF).
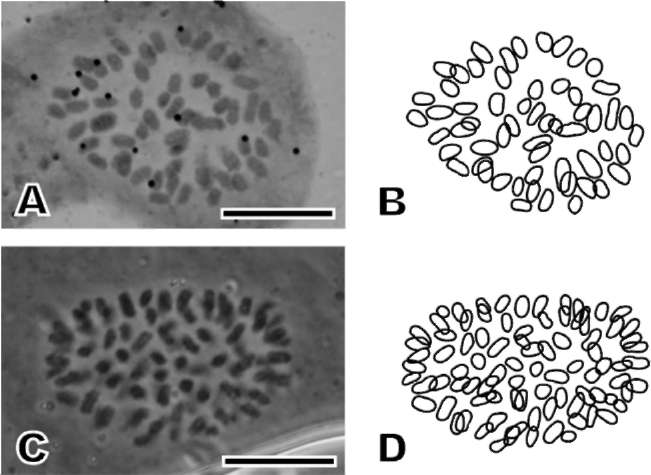
To count chromosomes, root tips were pretreated with 70 ppm cycloheximide and 250 ppm 8-hydroxyquinoline (1:1) at 18-20°C for 8 h. They were fixed sequentially in 45% acetic acid and absolute ethanol (1:3) overnight and preserved in 70% ethanol at 4°C. Then they were macerated in 1 N HCl at 60°C for 10 s, and digested in 4% pectinase for 1-2 h (Sharma, 1982; Huang et al., 2006).
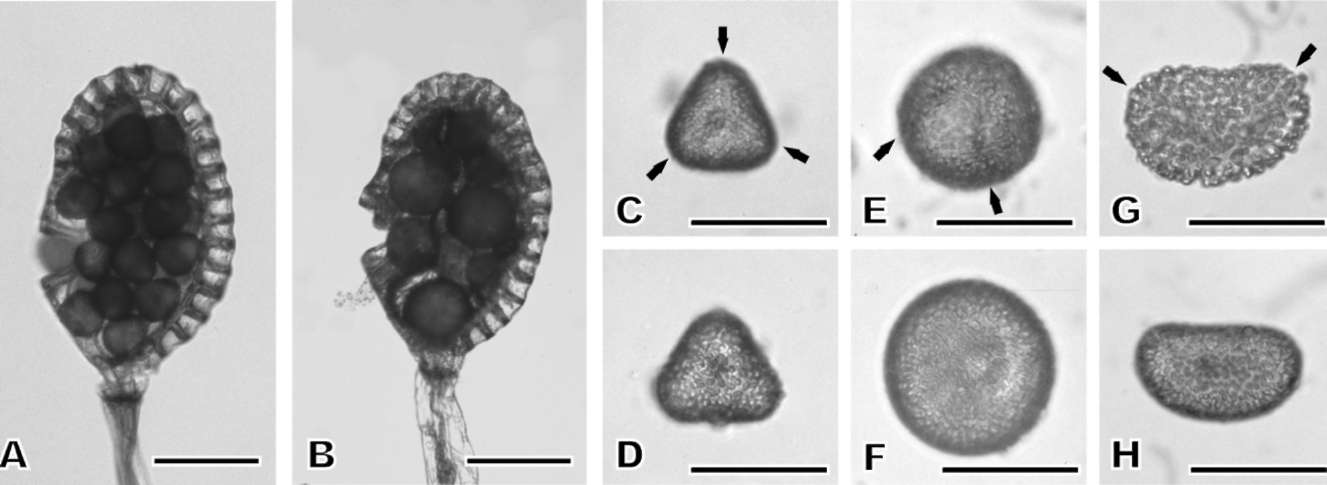
To count the number of spores per sporangium, five mature sporangia were picked randomly from each plant. Spores, including shrunk ones, were counted, but debris was excluded. To determine the spore size, fertile fronds were air-dried for seven days to release their spores. Then,
100 spores per plant, excluding shrunk ones, were chosen randomly. The shape and size (equatorial diameter, or length of the long axis) of each spore were recorded. The mean and standard deviation were calculated for each spore shape. One-way Analysis of Variance (ANOVA) followed by Tukey post-hoc test (p < 0.05) was used to compare the spore sizes of individuals.
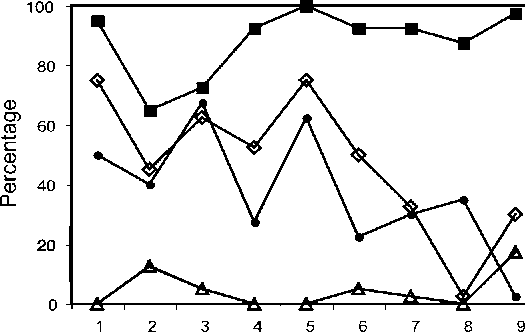
Germination rates were determined using the methods of Ko et al. (2004) and Huang et al. (2006). Spores of each plant were sown on two pieces of filter paper (pore size 0.45 fim, 47 mm in diameter, Gelman). Each piece of filter paper was placed in a box on a tile lying on the culture medium (vermiculite : peat : perlite = 4:4:2) for 4 weeks. Two hundred spores were sampled randomly from each piece of filter paper. A total of 400 spores from each plant were classified as germinated or ungerminated. Cultures were kept at 20-28°C under white fluorescent illumination (about 24 fimol m-2 s-1, 12 hd-1). After the germination rate had been determined, filter papers and tiles were removed and the gametophytes were remained and cultivated in the medium. After cultivation for three months, 40
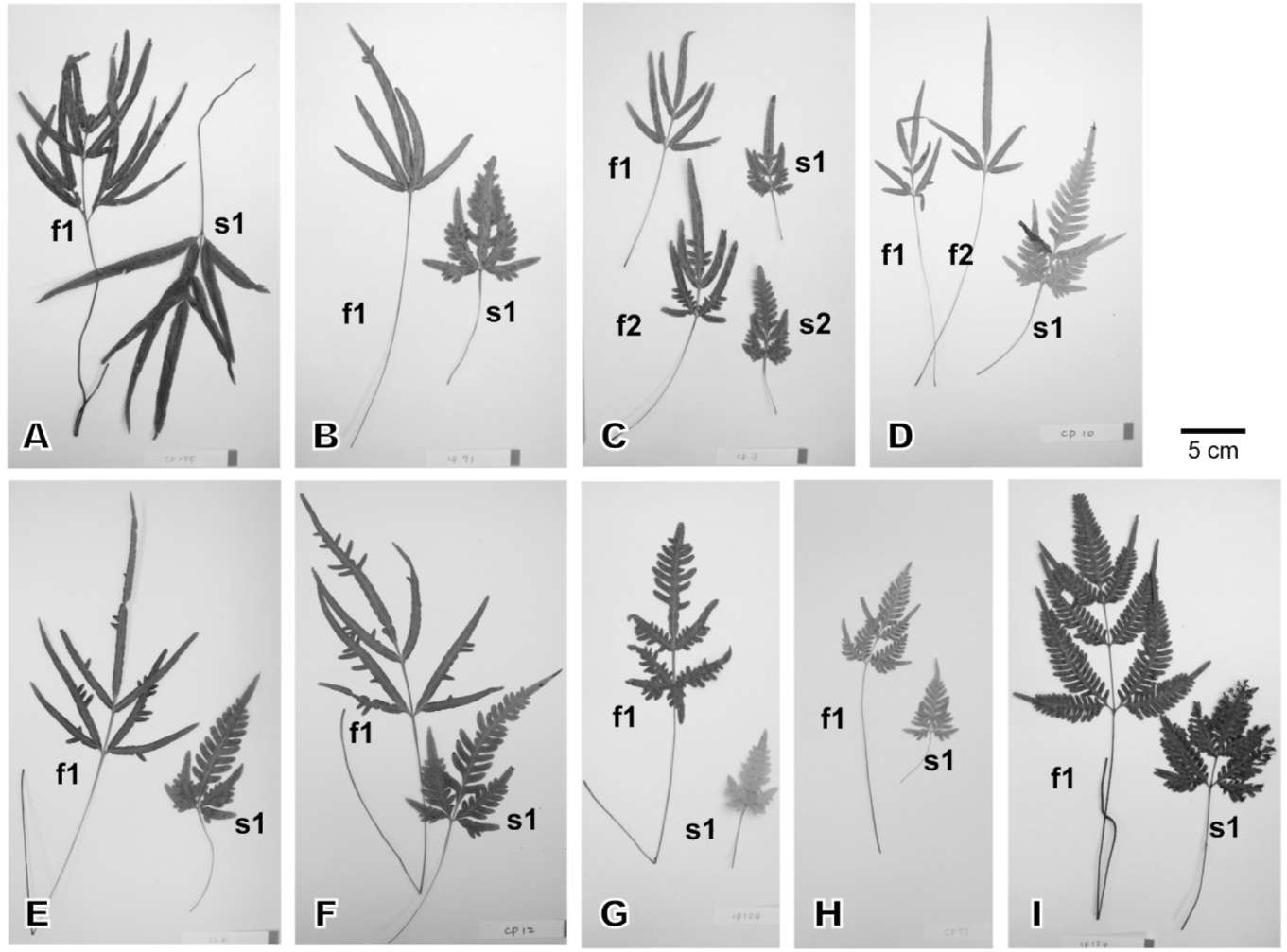
Figure 1. Photographs of the fronds of the 9 plants studied. A-G, representing plant 1-7, respectively, are Pteris cadieri; H & I, representing plant 8 & 9, respectively, are P. grevilleana. Two to four fronds from each plant were photographed. Fronds on the left are fertile (f1 and f2); those on the right are sterile (s1 and s2). The frond morphology of plants 1-9 varies along a continuum, from simply pinnate, irregularly bipinnatifid, to regular bipinnatifid.