HSIEH et al. — Antioxidants and antidiabetic effects of four Flemingia species 297
The correlation coefficients (R2) of the FRAP and TEAC assays conducted on the aqueous extracts of the four Flemingia species were shown in Figure 1. The R2 values of FRAP and TEAC assay showed high correlation (R2=0.83).
Scavenging activity against 1, 1-diphenyl-2-picrylhydrazyl radicals
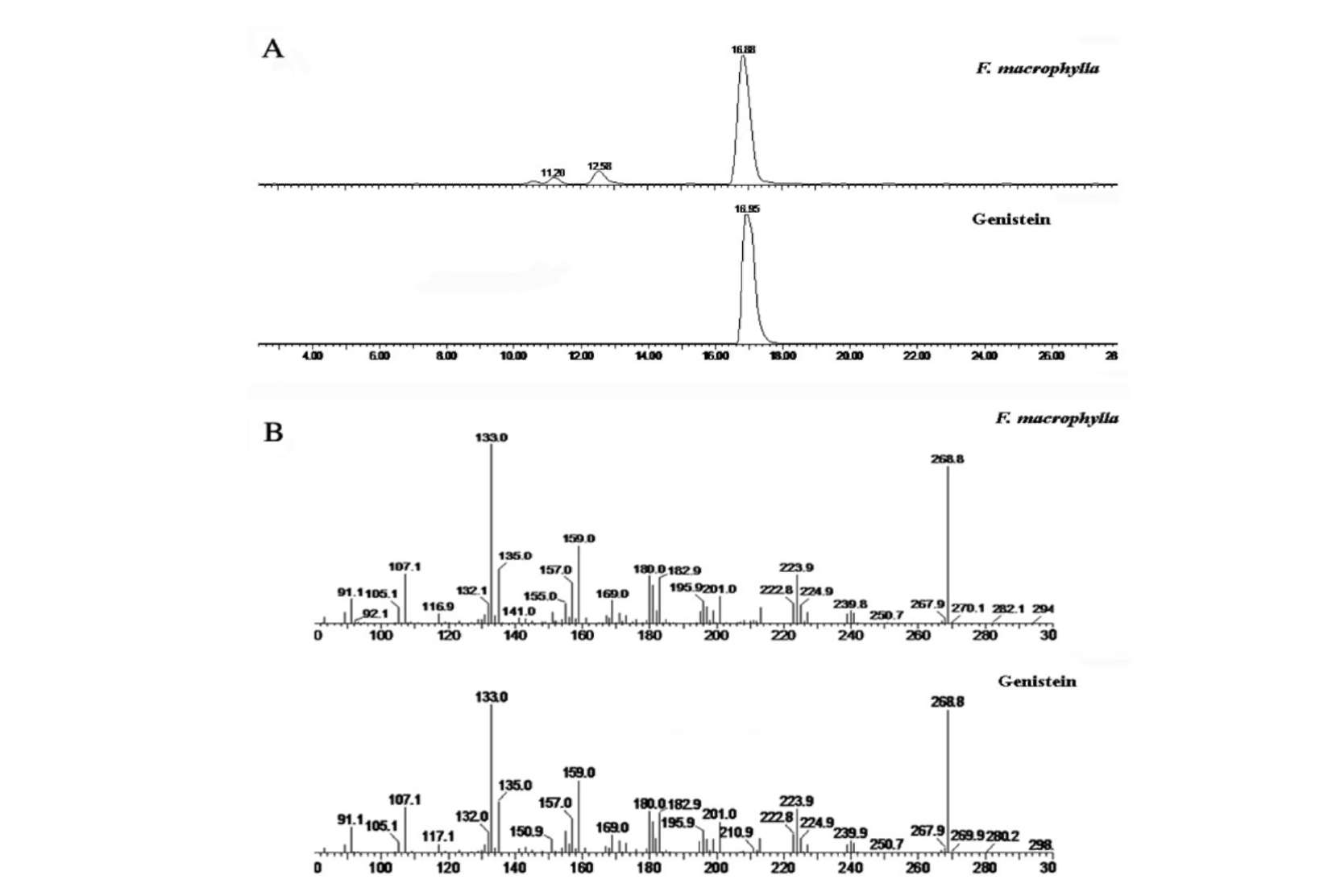
The relatively stable organic DPPH radicals are widely used in model systems to investigate the scavenging activities of several natural compounds, such as phenolics and anthocyanins, or crude mixtures. A DPPH radical is scavenged by antioxidants through the donation of a proton to form the reduced DPPH. The color changes from purple to yellow after reduction, which could be quantified by its decrease of absorbance at wavelength 517 nm. Radical scavenging activity increases when the percentage of free radical inhibition increases. Table 3 shows the IC50 values for the radical-scavenging activities of the four Flemingia species, GSH, and genistein using the DPPH colorimetric method. It was found that WFM had the lowest IC50 value (36.34 ± 1.22 μg/mL), followed by WFL (187.34 ± 1.28), WFS (197.97 ± 1.41 μg/mL), WFP (204.76 ± 0.57 μg/mL). The four extracts showed significant differences (p<0.05) in radical-scavenging activity. As demonstrated by the above results, the most active sample was WFM, however, its antioxidant capacity was still stronger than GSH and genistein positive controls (71.77 ± 1.39 μg/mL and 368 ±5.35 μg/mL) in DPPH assay.
Total polyphenol, flavonoid, and flavonol content
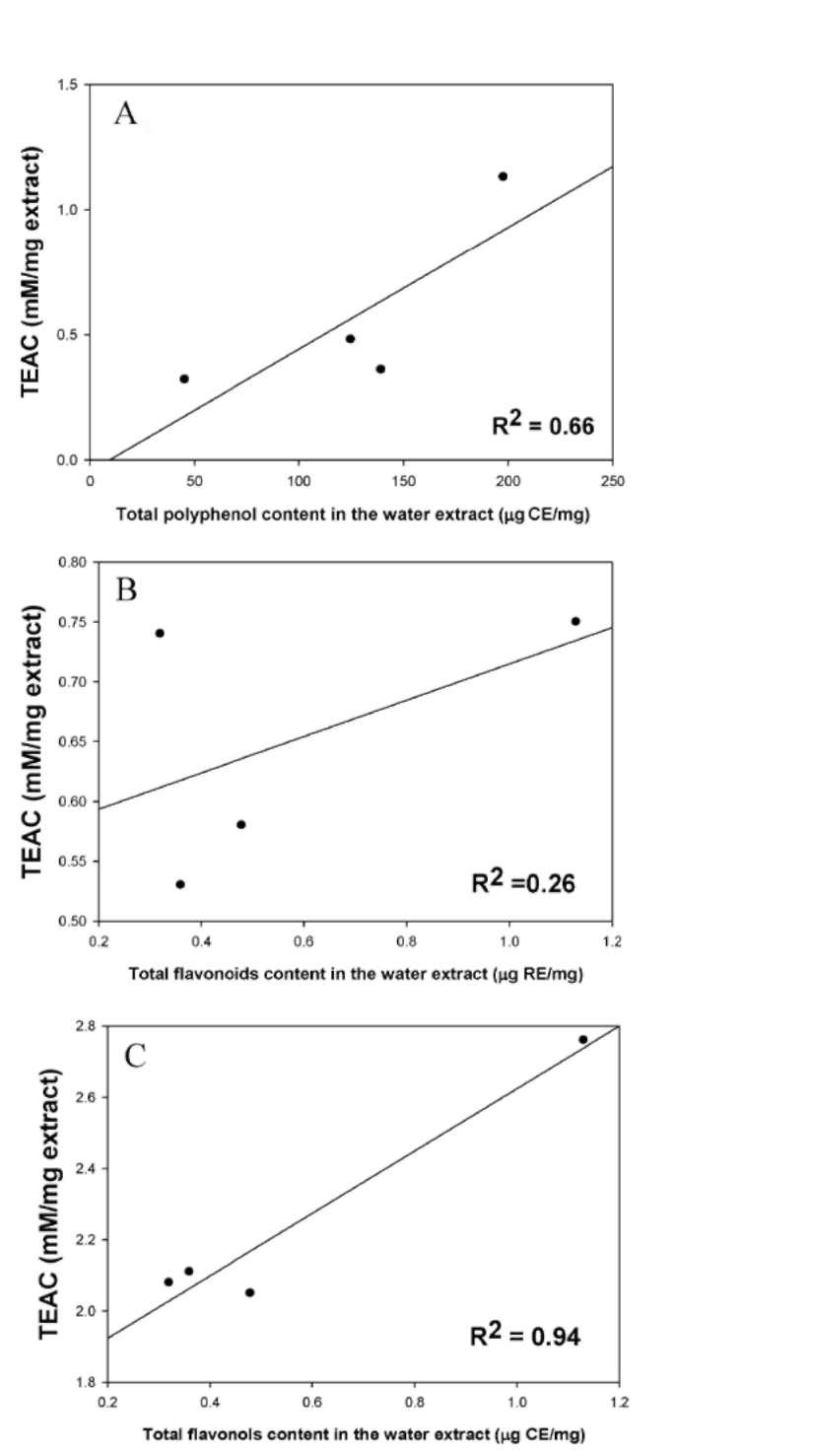
The total polyphenol, flavonoid, and flavonol contents of the four Flemingia species are shown in Table 4. The total polyphenol content was expressed as μg of catechin equivalent per milligram of dry weight. The total polyphenol contents of the extracts of the four Flemingia species ranged from 45.46 to 197.73 μg CE/mg, and
Table 3. IC50values of the aqueous extracts of four Flemingia species in DPPH radical scavenging activity.
Species and positive controls IC50(μg/mL)a
|
WFL
|
187.34 ± 1.28
|
||
|
WFM
|
36.34 士 1.22
|
||
|
WFP
|
204.76 ± 0.57
|
||
|
WFS
|
197.97 ± 1.41
|
||
|
GSH
|
71.77 ± 1.39
|
||
|
Genistein
|
368 士 5.35
|
||
aValues represent means ± S.D. of three parallel measurements.
Table 4. Contents of phytochemicals extracted from the aqueous extracts of four Flemingia species.
Species Total phenolsa Total flavonoidsb Total flavonolsa p (μg CE/mg) (μg RE/mg) (μg CE/mg)
|
WFL
|
139.42 士 1.59
|
0.53 士 0.02
|
2.11 士 0.10
|
||
|
WFM
|
197.73 ± 1.05
|
0.75 ± 0.04
|
2.76 ± 0.64
|
||
|
WFP
|
125.00 士 0.58
|
0.58 士 0.01
|
2.05 士 0.04
|
||
|
WFS
|
45.46 士 0.18
|
0.74 ± 0.04
|
2.08 士 0.04
|
||
*All data are expressed as means ± S.D. of triplicate tests. (n =
a 3) (p < 0.05).
aData expressed in (ig catechin equivalent/mg dry weight (μg
CE/mg).
bData expressed in μg rutin equivalent/mg dry weight (μg rutin/mg).
decreased in the following order: WFM > WFL > WFP > WFS. WFM had the highest polyphenolic content.
The total flavonoid contents were expressed as μg of rutin equivalent per milligram of dry weight. The total flavonoid contents of the extracts of the four Flemingia species ranged from 0.53 to 0.75 μg RE/mg, and decreased
in the following order: WFM > WFS > WFP > WFL.
WFM had the highest flavonoid content.
The total flavonol contents were expressed as μg of catechin equivalent per milligram of dry weight. The total flavonol contents of the extracts of the four Flemingia species ranged from 2.05 to 2.76 μg CE/mg, and decreased
in the following order: WFM > WFL > WFS > WFP.
WFM had the highest flavonols.
Both flavonoid and flavonol are polyphenolic compounds. Polyphenolic compounds have important roles in stabilizing lipid oxidation and are associated with antioxidant activities (Yen et al., 1993). The phenolic compounds may contribute directly to antioxidative actions (Duh et al., 1999). It is suggested that 1.0 g of polyphenolic compounds from a daily diet rich in fruits and vegetables has inhibitory effects on mutagenesis and carcinogenesis in humans (Tanaka et al., 1998). The antioxidative activities observed could be ascribed both to the different mechanisms exerted by various phenolic compounds and to the synergistic effects of different compounds. The antioxidant assay used in
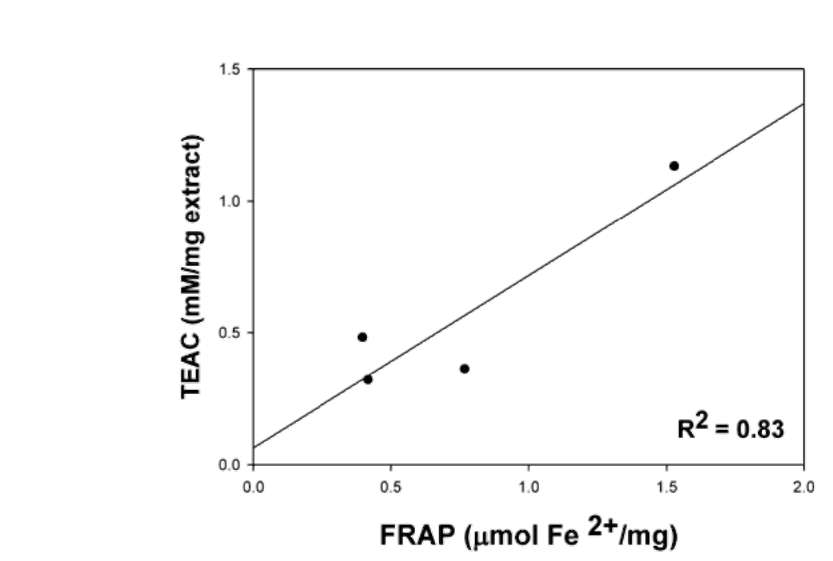
Figure 1. Correlation coefficients (R2) of TEAC and FRAP in the aqueous extracts of the four Flemingia species.