JU et al. ― Chemical constituents of Lacrymaria velutina
313
Nitrite measurement and cell viability assay
The methods were essentially the same as reported previously (Wang et al., 2007; Lee et al., 2008). To assess the effects on LPS-induced NO production, crude extracts of fermented broth of L. velutina, compounds 1-8, two positive controls-N^-nitro-L-arginine (L-NNA, a nonselective NOS inhibitor) and aminoguanidine (a specific inhibitor of iNOS)—or vehicle (0.1%, DMSO) were added in the presence of LPS (200 ng/ml) to the RAW 264.7 cells. Both inhibitors were purchased from Sigma-Aldrich Chemical Co. and the purity of each compound was more than 98%.
Statistical analyses
Comparisons of the concentration and treatment effects were made using ANOVA, followed by post hoc comparisons using Newman-Keuls test as appropriate. The average IC50 was determined by data fitting with GraFit (Erithacus Software, Staines, UK).
RESULTS AND DISCUSSION
The fermented broth of L. velutina was partitioned initially using EtOAc to give a brown residue, which was subjected to Sephadex LH-20 column separation followed by HPLC purification to obtain one new diterpenoid (1) along with 5-hydroxymethylfurfural (2) (McNelis et al., 1994), ^is-2,5-hydroxymethylfuran (3) (Fawcett et al., 1987), maltol (4) (Sun et al., 1995), succinic acid
(5) (Abdel-Farid et al., 2007), and furan-2,5-diol (6)
(Rappoporta et al., 2001). Meanwhile, the cultured mycelium of the same fungus was extracted with methanol, then partitioned using n-hexane to obtain a yellow residue, which was re-crystallized from n-hexane to afford a mixture of (22E,24S)-24-methyl-27-norcholesta-5,7,22-trien-3p-ol (7) and (22E,24R)-24-methyl-27-norcholesta-5,7,22-trien-3p-ol (8) (Itoh et al., 1983)/
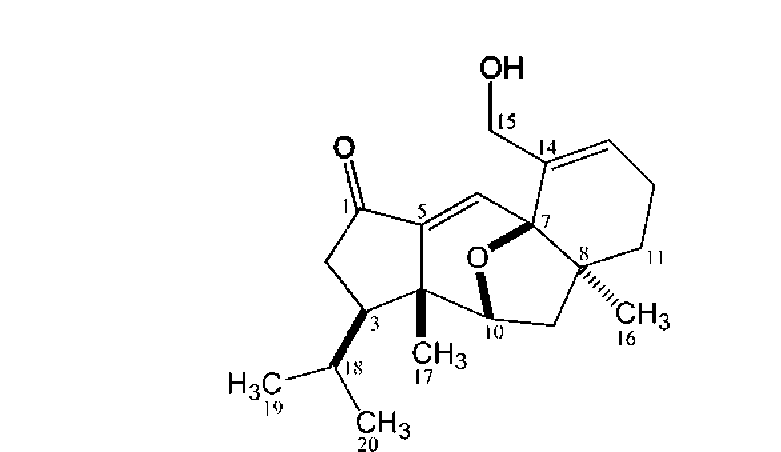
Compound 1 was isolated as light yellow oil, and its molecular formula, C20H28O3, was established through analysis of its 13C-NMR and HR-ESI-MS data. The IR spectrum of 1 exhibited the presence of a carbonyl group
(1,715 cm-1), a double bond (1,638 cm-1) and a hydroxyl group (3,370 cm-1). The interpretation of the 1H-NMR of 1 supported by its HSQC assignments revealed signals for four methyl groups [§h 0.78 (3H, d, J = 6.3 Hz, H3-20), §h 0.81 (3H, d, J = 6.3 Hz, H3-19), §h 0.89 (3H, s, H3-16) and §h 1.25 (3H, s, H3-17)], five methylene groups [§h 1.28 (1H, m, H-11a), §h 1.96 (1H, m, H-11b), §h 1.66 (1H, dd, J = 8.1, 13.0 Hz, H-9a), §h 1.82 (1H, dd, J = 2.2, 13.0 Hz, H-9b), §h 2.01, 2.09 (each 1H, m, H2-12), §h 2.14 (1H, dd, J = 11.8, 18.1 Hz, H-2a), §h 2.40 (1H, dd, J = 7.3, 18.1 Hz, H-2b), §h 4.53, 4.78 (each 1H, d, J = 13.7 Hz, &-15)] and five methine protons [§h 1.54 (1H, m, H-18), §h 1.59 (1H, m, H-3), §h 4.45 (1H, dd, J = 2.2, 8.1 Hz, H-10), §h 6.37 (1H, brd, J = 4.1 Hz, H-13), §h 6.85 (1H, s, H-6)] (Table 1). The 13C-NMR spectrum together with DEPT analysis displayed 20 signals including four methyl carbons, five methylene carbons, three methine carbons and three quaternary carbons in the aliphatic region, two methine carbons and two quaternary carbons in olefinic region (2 double bonds) as well as one carbonyl carbon (Table 1). On account of the molecular formula C20H28O3, the double bond equivalence of 1 was seven including one carbonyl and two olefinic functionalities. Thus, there should be four rings in 1. Analysis of COSY and HSQC spectral data allowed the assignments of three spin systems including an aliphatic chain [(H3-19, -20)-(H-18)-(H-3)-(H2-2)-], two two-resonance units [-(H-10)-(H2-9)- and -(H2-12)-(H-13)-] and five geminal coupled methylene functionalities
[-(H2-2)-, -(H2-9)-, -(H2-11)-, -(H2-12)- and -(H2-15)-].
In the HMBC spectrum, key long range proton-carbon correlations including H3-20 (§h 0.78)/C-19 (§c 22.4), -18 (§c 29.8), -3 (§c 46.2), H3-19 (§h 0.81)/C-20 (§c 22.2), -18 (§c 29.8), -3 (§c 46.2), H3-17 (§h 1.25)/C-10 (§c 81.5), -5 (§c 145.5), -4 (§c 46.4), -3 (§c 46.2), H3-16/C-H (§c 34.4), -9 (§c 40.4), -8 (§c 48.6), -7 (§c 80.5), H-10 (§h 4.45)/C-5 (§c 145.5), H-6 (§h 6.85)/C-7 (§c 80.5), -4 (§c 46.4), -1 (§c 203.7) and H-2b (§h 2.40)/C-5 (§c 145.5), -4 (§c 46.4), -3 (§c 46.2), -1 (§c 203.7) established the major connectivities of the fragments deduced from COSY spectrum. All above assignments suggested that the plane structure of 1 seemed to be analogous to guanacastepenes, unique diterpenoids with a 5/7/6-fused ring system, isolated previously from an unidentified endophytic fungus (Brady et al., 2000, 2001). However, the conspicuous cross peak between carbinoyl H-10 (§h 4.45) and oxygenated quaternary C-7 (S。 80.5) in the HMBC spectrum of 1 suggested that C-10 and C-7 were bridged by an ether functionality, forming a different 5/6/5/6-fused ring skeleton of 1. In the NOESY spectrum of 1, key mutual correlations were listed as follows: H3-
20/H3-17, H3-17/H-10, H-10/H-9a and H-9b/H3-16, which
were further supported by the three dimensional molecular modeling under minimized energy condition (Figure 1). The relative configurations of the isopropyl group attached at C-3, H3-17, ether linkage between C-7 and C-10, and H3-16 were thus corroborated to be respective p, p, p and a oriented to fit the distinguishing features in NOESY spectrum (Table 1). Accordingly, 1 was characterized as the shown structure (Figure 2), and named lacrymarone.
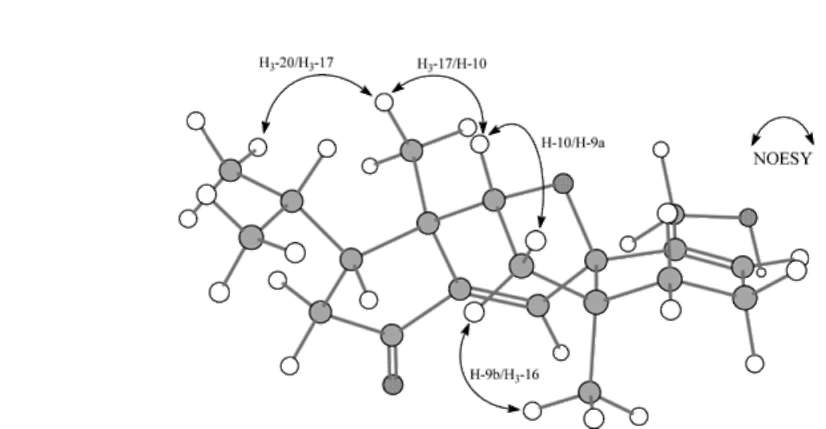
Figure 1. Computer-generated perspective drawing for 1, which accommodate observed mutual key NOESY