326
Botanical Studies, Vol. 51, 2010
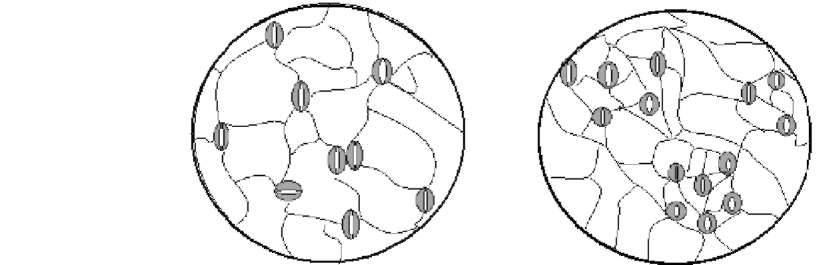
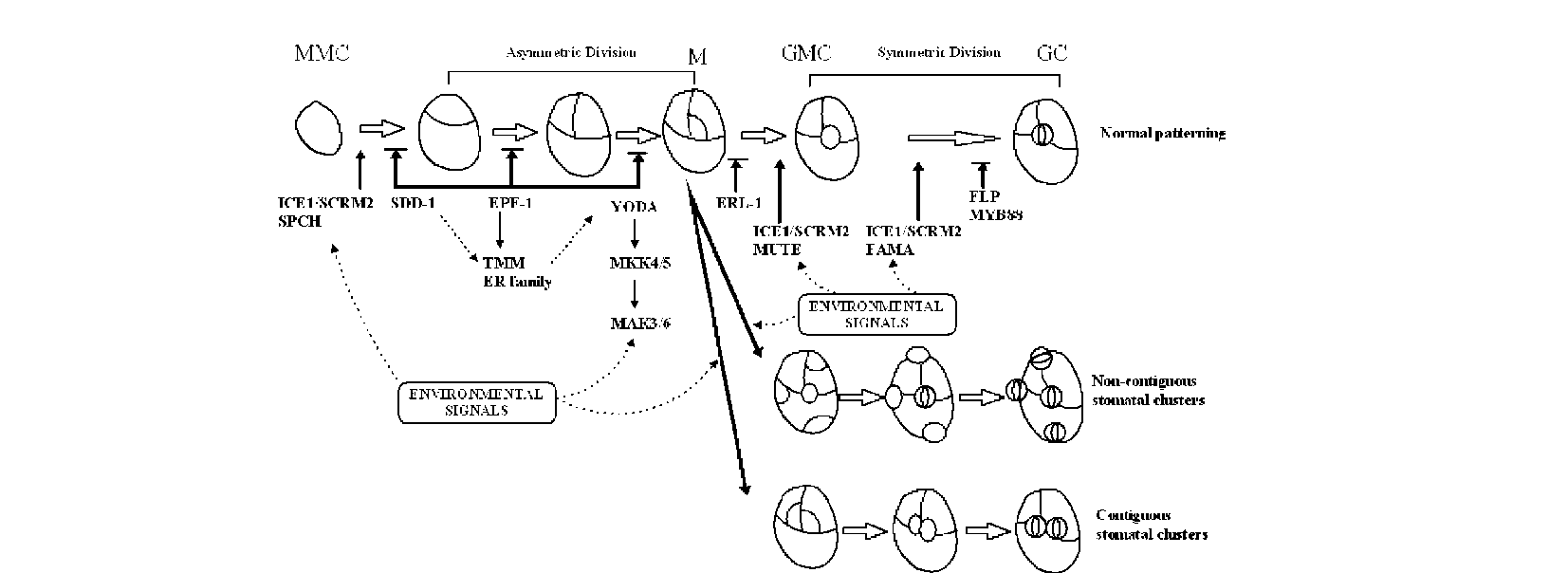
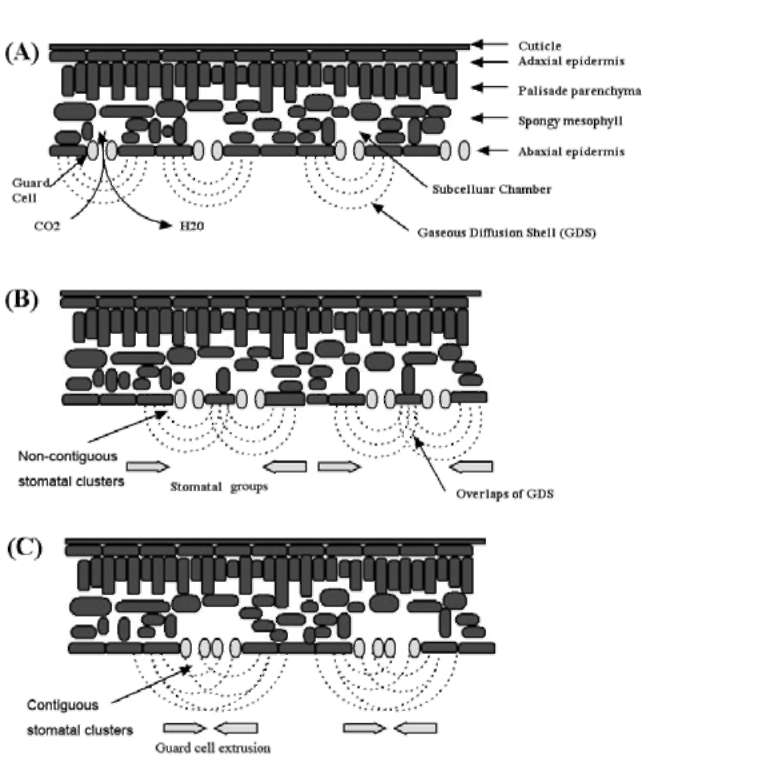
These two types of clusters are still not well classified: the term "stomatal cluster" has been used for both types in many studies (Sachs, 1994; Tang et al., 2002; Zhao et al., 2006). Therefore, a clear definition and classification must be redrawn. In the present study, we collected the leaves of 16 plant species which contain normal stomatal patterning and the two types of clusters. Then we used the classical methods which were developed to assess stomatal distribution in ecological studies (Clark and Evans, 1954; Korn, 1993) to evaluate the difference between thesetwo stomatal cluster types.
Interestingly, many plants found to have stomatal clusters were living in arid, salty, or otherwise adverse environments: laterally contiguous stomata were found on the epidermis of Melilotus suaveolens Ledeb., which was sampled on the beach at Hangzhou Bay (Figure 1D). Similar clusters were also reported in a typical halophyte Sonneratia alba J. Smith (Sonneratiaceae) (Chen, 1996). Sedum dfredii Hance, which exhibits stomatal clusters, is a typical CAM plant with strong stress tolerance (Payne, 1970). However, fewer studies have been conducted to address questions like why so many plants bear stomatal
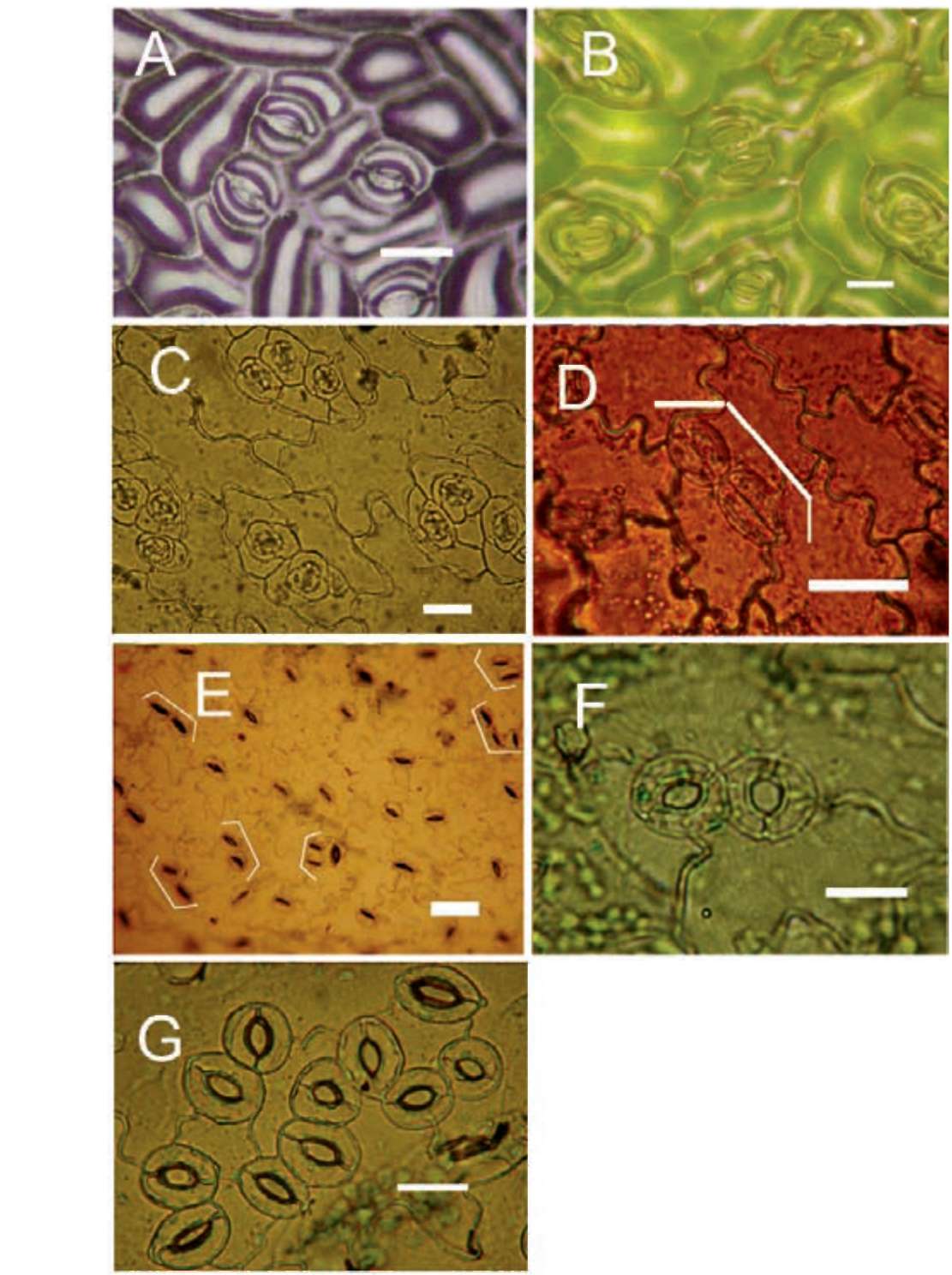
Figure 1. Stomatal clustering in various plant species: (A) Nail polisher imprint show laterally contiguous stomata in Cinnamo-mum camphora (Lauraceae); (B) Non-contiguous stomatal clusters in Rieger begonia (Begoniaceae); (C) Non-contiguous stomatal clusters in Sedum dfredii Hance (Crassulaceae); (D) Polarly contiguous stomata occasionally seen in Melilotus sua^veolens Ledeb (Leguminosae); (E) Contiguous stomatal clustering in the leaf epidermis of Vicia faba Linn. (Le-guminosae) under water stress; (F) Laterally contiguous stomata occasionally seen in Arabidopsis thaliana L. (Brassicaceae) Col-0 ecotype; (G) Large contiguous stomatal clusters in the leaf epidermis of Arabidopsis mutant line too many mouths. Stomatal clusters were marked with white cases. Bars in the pictures represent for 25 |im.