358
Botanical Studies, Vol. 51, 2010
gelrite (4,000). Medium pH was adjusted to 5.2 before autoclaving for 15 min at 121°C. The seeds were sown in 20x150 mm Pyrex tubes each with 9 ml GM medium.
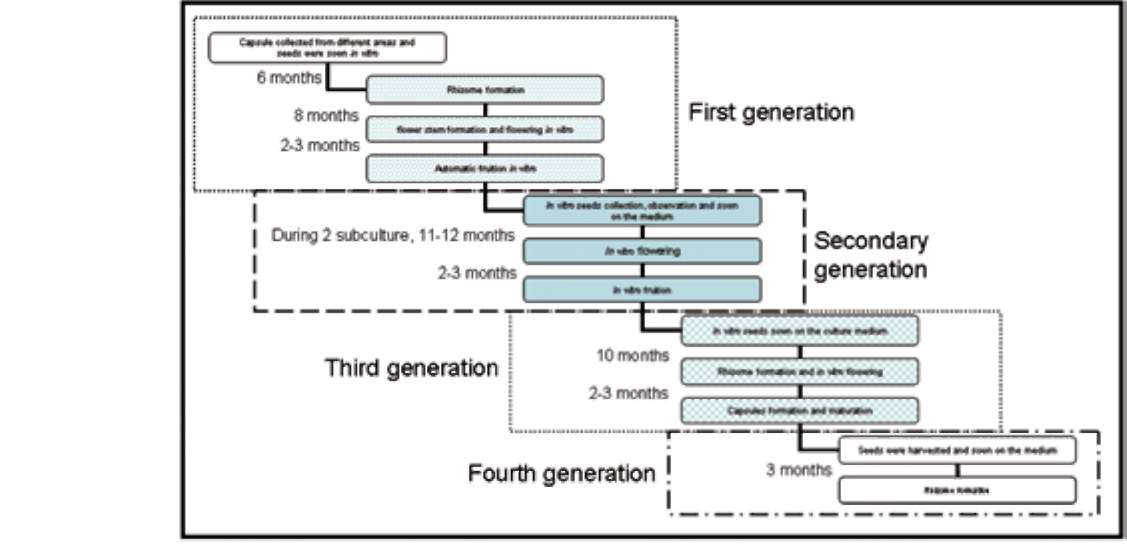
Protocorms were subcultured in the same medium for rhizome development. The rhizomes were subcultured for a period between 4-6 months. All rhizome cultures were exposed to artificial light of 1000 lux (daylight fluorescent tubes FL-30D/29, 40 w, China Electric Co, Taipei, Taiwan) with an average light/dark cycle of 16/8 h at 25 士 1°C.
In vitro fruit set.
Plantlets with a 1-cm pseudobulb of E. graminea were transferred to the induction medium for fruit setting (FM). The medium formula was WPM basal salts (Lloyd and McCown, 1981) with (mg l-l): myo-inositol (100), activated charcoal (1,000), BA (1.0), NAA (0.6), sucrose (20,000) and solidified with agar (7,000). The medium pH was adjusted to 5.2. The in vitro capsules were collected and seeds were sown on the GM medium.
All statistical analyses were carried out using One-way ANOVA Duncan's multiple range test at a 95% confidence level with the COSTAT statistical software.
Scanning electron microscopy
Samples for scanning electron microscopy were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 4 h, then dehydrated using an ethanol series, dried in a critical-point dryer (HCP-2, Hitachi, Japan), and finally coated with gold using an ion coater (E1010, Hitachi, Japan)
before viewing with a Hitachi S-3000N scanning electron microscope (Chang et al., 2005).
In vivo pollen tube growth
The style of in vitro self-pollinated flowers were collected and fixed in FAA (50% ethanol : acetic acid : formaldehyde = 18:1:1) for 24 h, rinsed in demineralized water, and softened in 3 M NaOH for 2 h at 60°C. Afterwards, the style were rinsed in demineralized water and placed in a drop of aniline blue solution with 2% glycerol, squashed under a cover slip. After staining, it was observed by fluorescence microscopy (Peter et al., 2004).
results
Seed germination and plantlet establishment
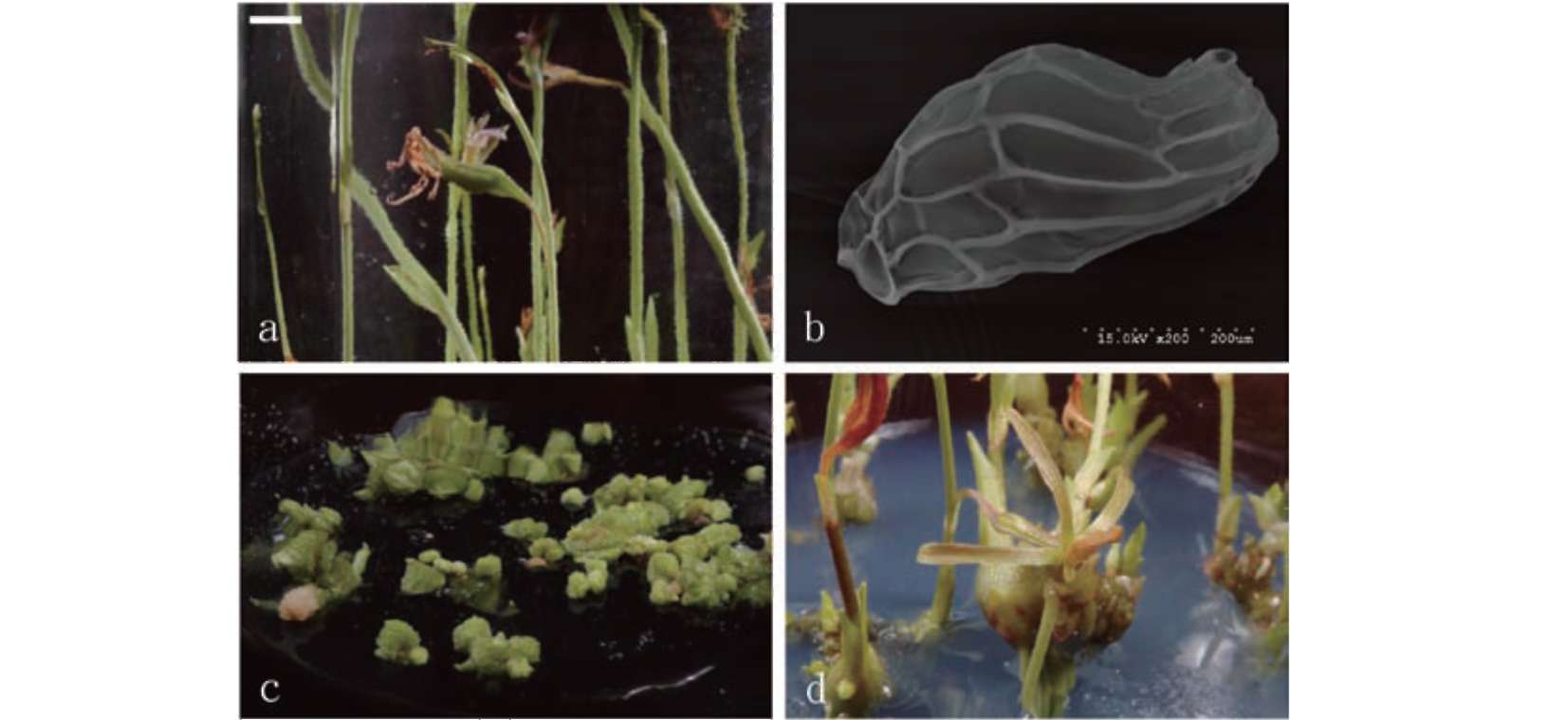
Seeds of E. graminea (Figure 1a) were sown in vitro. After 2-3 months, the embryos swelled and broke out of the testa, and then formed protocorms (Figure 1b). The protocorm enlarged, and produced rhizomes with multiple buds (Figure 1c). The six month old rhizomes were subcultured in the same medium and then subcultured again for 4 months. The apex of the rhizome formed either vegetative buds (Figure 1d) or flower stems (Figure 1e). After the vegetative buds grew into a complete plantlet with expanded leaves, and were transplanted in a greenhouse where they survived and were healthy (Figure 1f). Subsequently, several plants were blooming the following year.

Figure 1. The seed germination, rhizomes development, plantlet establishment and flowering in vitro of Eulophia graminea. (a) Seed (bar = 70 (im); (b) Protocroms (bar = 100 (im); (c) Rhizomes (bar = 0.60 mm); (d) Shoots derived from rhizomes (bar = 1.3 cm); (e) Flower stems derived from rhizomes (bar = 6 mm); (f) Healthy seedling grown in greenhouse (bar = 1.9 cm).