374
Botanical Studies, Vol. 51, 2010
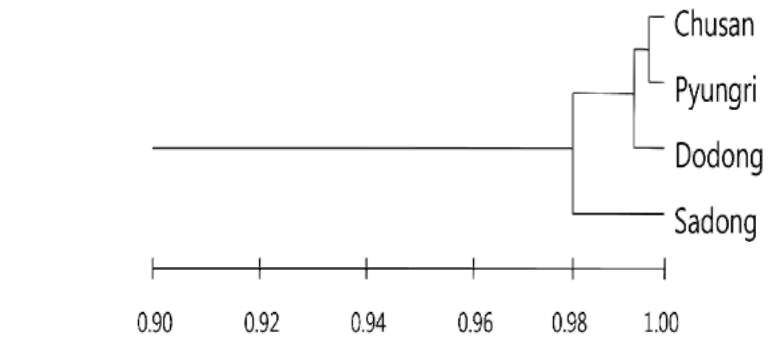
of all, four populations of S. takesimensis had a low FST value, which indicates a close similarity between subpopulations. However, S. takesimensis also showed a large deficiency of heterozygotes, which directly indicates ongoing, intensive inbreeding, which must result in high differentiation among subpopulations (Table 5). These seemingly contradictory results of a low FST value and heterozygote deficiency can be explained by hypothesizing that although S. takesimensis is currently undergoing intensive inbreeding, it has not had much time yet to differentiate among subpopulations since arriving at Ulleung Island.
In addition, the fact that Ulleung Island is closer to the mainland than other islands such as Hawaii or Bonin adds support to the aforementioned hypothesis. The closer an island is to the mainland, the more likely it is that it will receive seeds or living individuals from there, which can possibly introduce a new allele to the insular gene pool, increasing the genetic variation as time passes by. These kinds of explanations were well supported in case of Campanula takesimana which is also a species endemic to Ulleung Island (Park and Jung, 2000). The relatively high genetic variation of the species suggested that it may be originate from multiful genetically polymorphic progenitor populations.
However, the S. takesimensis shows low genetic variation even though Ulleung Island is close to mainland Korea. This obviously indicates that there has not been enough time for mainland individuals to cross the East Sea and migrate there, which supports the short insular history of S. takesimensis. This could be also explained by postulating that S. takesimensis arrived at Ulleung Island by a single introduction from remote regions via longdistance dispersal rather than from the Korean mainland. One possible scenario is that S. takesimensis originated from Japanese endemic S. grayanoides, which has a limited distribution in the northeastern part of Honshu (Kamada et al., 2007). Recent molecular phylogenetic analysis using ITS data showed the sister-group relationship between S. takesimensis and S. grayanoides (M. Kim, unpublished data).
Another possible reason for the extraordinarily low genetic variation of S. takesimensis is fragmentation of population. Despite the already small number of individuals of S. takesimensis, they are divided into even smaller subpopulations throughout Ulleung Island. Since small populations caused by habitat fragmentation tend to inbreed and are affected by genetic drift, smaller subpopulations undergo even more intense inbreeding and genetic drift, resulting in a significant reduction in the number of heterozygotes, which ultimately lowers the genetic variation of S. takesimensis (Neel and Ellstrand,
2001).
To effectively conserve S. takesimensis and prevent future extinction, the population size should be increased to a certain amount in an effort to reduce their vulnerability to possible environmental changes. Furthermore, a larger
Figure 1. UPGMA phenogram derived from Nei's genetic identity of four populations of S. takesimensis in Ulleung Island, Korea.
DISCUSSION
Genetic variation is the most influential value in determining survival of a population or a species in both the long and short term. A decrease in heterozygosity results in reduced individual and population viability, and a drop in allelic diversity significantly undermines a species' ability to acclimate to changing selection (Huenneke, 1991). Hence, being fully aware of genetic diversity and its distribution is the first and foremost step in setting conservation plan for endangered species such as S. takesimensis.
According to much genetic diversity research, endemic island species generally have lower genetic variation than related continental species (Park and Jung, 2000). In the case of the Bonin Islands (Ito et al., 1998), for example, endemic species belonging to genus Crepidiastrum, Pittosporum, and Symplocos showed very low heterozygosity compared to continental species. Additionally, in the case of Hawaiian endemic species (Dejoode and Wendel, 1992), mean alleles per locus of 64 species were 1.32, and the percentage of heterozygotes of endemic island angiosperms was found to be 0.25. Because of a small founder population and a severe bottleneck effect and inbreeding, endemic island species display significantly low genetic variation (Ito et al.,
1998).
Scrophularia takesimensis, an endangered endemic island species, shows very low genetic variation. In comparison with other island species such as Hawaiian and Bonin species, S. takesimensis has relatively low genetic variation. In the case of Hawaiian endemic species (B. insignis and B. rockii) (Gemmill et al., 1998), the mean number of alleles per locus was 1.3, and their percentage of polymorphic loci was 22.8%. Additionally, the expected heterozygosity was 0.040. However, S. takesimensis showed even lower genetic variation, with the mean number of alleles per locus being 1.1 and their percentage of polymorphic loci being 11.1% while the expected heterozygosity was only 0.028. The most probable reason for this aberrational result is the recent introduction of S. takesimensis to Ulleung Island. First