|
|
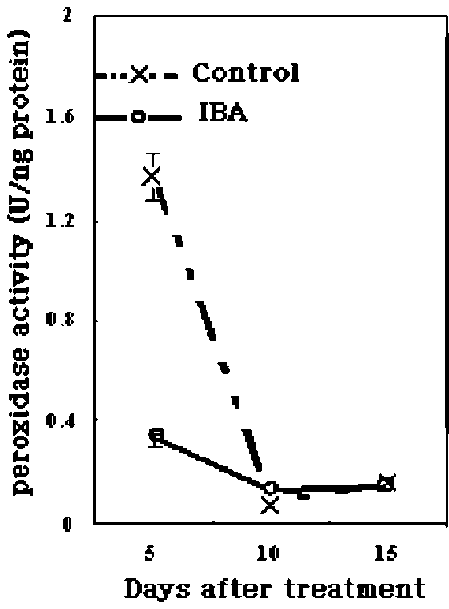
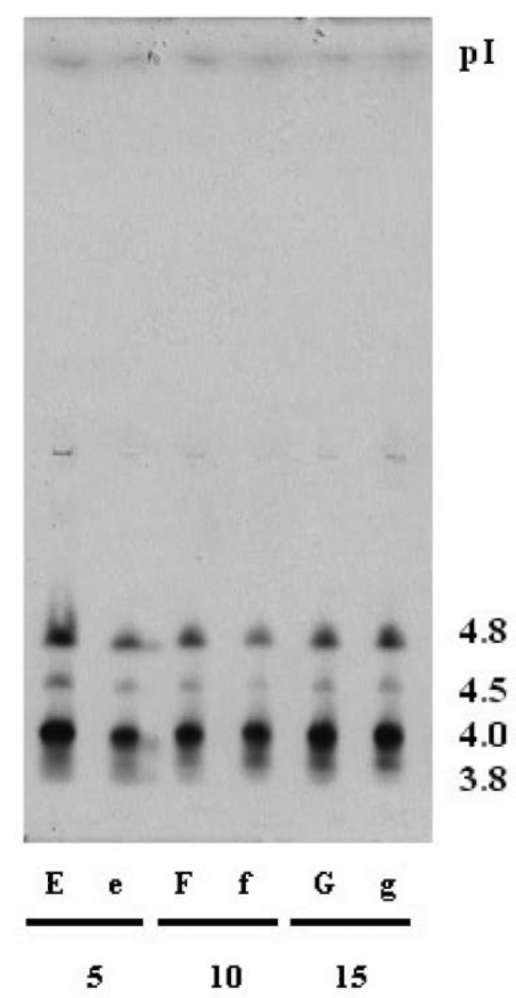
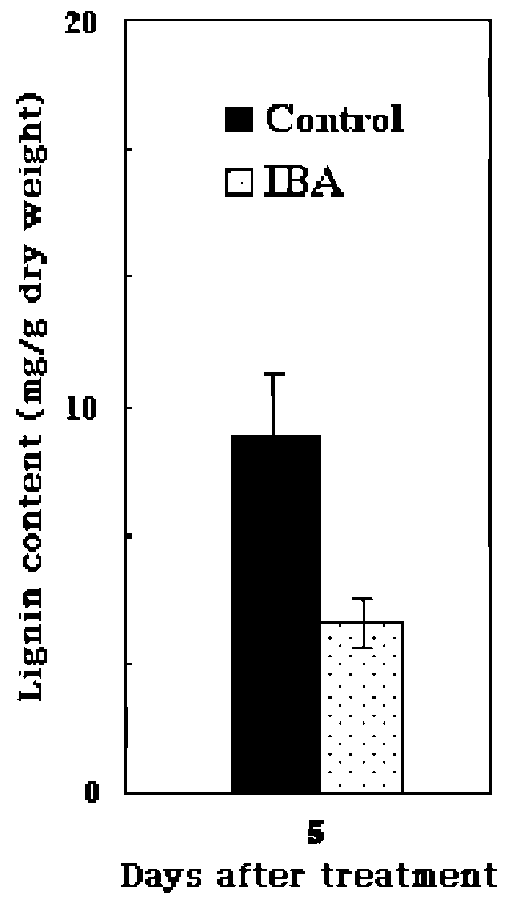
metabolism, lignin and suberin formation, and defense against pathogens, (Klotz and Lagrimini, 1996; Passardi et
al., 2005).
Acknowledgments. Funding (NSC98-2621-B-110-001) of this work by the National Science Council, Republic of China is gratefully acknowledged.
LITERATURE CITED
Aeschbacher, R.A., J.W. Schiefelbein, and P.N. Benfey. 1994. The genetic and molecular basis of root development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 25-45.
Beffa, R., H.V. Martin, and P.E. Pilet. 1990. In vitro oxidation of
indoleacetic acid by soluble auxin-oxidases and peroxidases from maize root. Plant Physiol. 94: 485-491.
Bruce, R. and C.A.West. 1989. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 91: 889-897.
Carpin, S., M. Crevecoeur, H. Greppin, and C. Penel. 1999. Molecular cloning and tissue-specific expression of an anionic peroxidase in zucchini. Plant Physiol. 120: 799-810.
Chao, I.L., C.L. Cho, L.M. Chen, and Z.H. Liu. 2001. Effect of
indole-3-butyric acid on the endogenous indole-3-acetic acid and lignin contents in soybean hypocotyl during adventitious root formation. J. Plant Physiol. 158: 1257-1262.
Chen, L.M., J.T. Cheng, E.L.Chen, T.J. Yiu, and Z.H. Liu.
2002. Naphthaleneacetic acid suppresses peroxidase activity during the induction of adventitious root in soybean hypocot-yls. J. Plant Physiol. 159: 1349-1354.
Chou, C.H., Y.C. Huang, and Z.H. Liu. 2010. Peroxidase genes differentially respond to auxin during the formation of adventitious roots in soybean hypocotyls. Plant Growth Regul. 60: 151-161.
Christensen, J.H., G. Bauw, K.G. Welinder, M.V. Montagu, and W. Boerjan. 1998. Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiol. 118: 125-135.
Christensen, J.H., S. Overney, A. Rohde, W.A. Diaz, G. Bauw, P. Simon, M.V. Montagu, and W. Boerjan. 2001. The syrin-galdazine-oxidizing peroxidase PXP 3-4 from poplar xylem: cDNA isolation, characterization and expression. Plant Mol. Biol. 47: 581-593.
Da Costa e Silva, O., L. Klein, E. Schmeizer, G.F. Trezzini, and
K. Hahlbrock. 1993. BPF-1, a pathogen-induced DNA-
binding protein involved in the plant defense response. The
Plant J. 4: 125-135.
Ditengou, F.A., W.D. Teale, P. Kochersperger, K.A. Flittner, I. Kneuper, E. V. D. Graaff, H. Nziengui, F. Pinosa, X. Li, R. Nitschke, T. Laux, and K. Palme. 2008. Mechanical induction of lateral root initiation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 18818-18823.
Dubrovsky, J.G., M. Sauer, S. N. Mendivil, M.G. Ivanchenko, J. Friml, S. Shishkova, J. Celenza, and E. Benkova. 2008. Auxin acts as a local morphogenetic trigger to specify later-
|
al root founder cells. Proc. Natl. Acad. Sci. USA 105: 87908794.
Gajhede, M., D.J. Schuller, A. Henriksen, A.T. Smith, and T.L.
Poulos. 1997. Crystal structure of horseradish peroxidase C at 2.15 A resolution. Nature Structural Biol. 4: 1032-1038.
Gaspar, T., C. Kevers, J.F. Hausman, J.Y. Berthon, and V. Rip-etti. 1992. Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie 12: 757-765.
Gazaryan, I.G., T.A. Chubar, E.A. Mareeva, L.M. Largimini, R.B. Vanhuystee, and R.N.F. Thorneley. 1999. Aerobic oxidation of indole-3-acetic acid catalyzed by anionic and cationic peanut peroxidase. Phytochemistry 51: 175-186.
Grotewold, E., B.J. Drummond, B. Bowen, and T. Peterson. 1994. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76: 543-553.
Habberer, G., M.T. Mader, P. Kosarev, M. Spammagl, L. Yang, and K.E. Mayer. 2006. Large-scale cis-Element detection by analysis of correlated expression and sequence conservation between Arabidopsis and Brassica oleracea. Plant Physiol. 142: 1589-1602.
Hiraga, S., K. Sasaki, H. Ito, Y. Ohashi, and H. Matsui.
2001. A large family of class III plant peroxidases. Plant Cell
Physiol. 42: 462-468.
Ito, H., S. Hiraga, H. Tsugawa, H. Matsui, Y. Otsuki, T. Murakami, and Y. Ohashi. 2000. Xylem-specific expression of wound-inducible rice peroxidase genes in transgenic plants. Plant Sci. 155: 85-100.
Klotz, K.L. and L.M. Lagrimini. 1996. Phytohormone control of the tobacco anionic peroxidase promoter. Plant Mol. Biol.
31: 565-573.
Lagrimini, L.M., R.J. Joly, J.R. Dunlap, and T.T. Liu. 1997. The
consequence of peroxidase overexpression in transgenic plants on root growth and development. Plant Mol. Biol.
33: 887-895.
Lanteri, M.L., A.M. Laxalt, and L. Lamattina. 2008. Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during Auxin-induced adventitious root formation in cucumber. Plant Physiol. 147: 188-198.
Musel, G., T. Schindler, R. Bergfeld, K. Ruel, G. Jacque, C.
Lapierre, V. Speth, and P. Schoptfer. 1997. Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical and immunological
probes. Planta 201: 146-159.
Nigel, C.V. 2004. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65: 249-259.
Passardi, F., C. Cosio, C. Penel, and C. Dunand. 2005. Peroxi-dases have more functions than a Swiss army knife. Plant
Cell Rep. 24: 255-265.
Peret, B., B.D. Rybel, I. Casimiro, E. Benkova, R. Swarup, L. Laplaze, T. Beeckman, and M.J. Bennett. 2009. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 14: 399-408.
|
|