LIN et al. ― Bisulfite induced oxidative stress in rice seedlings
175
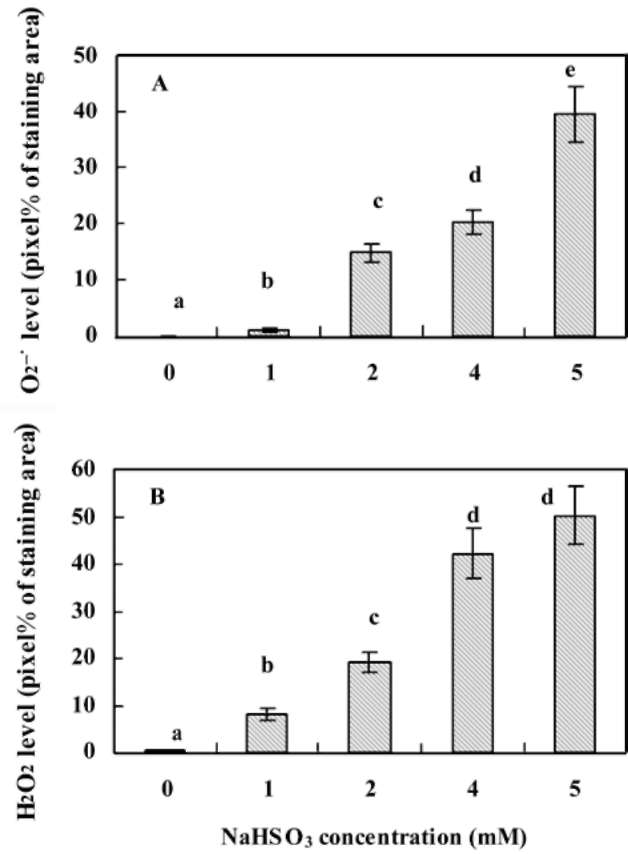
servation of and H2O2 generation in rice leaves in situ. When NBT, a specific probe of , is treated with O;, a dark blue formazan stain appears at the plant tissue's generation site. Increased concentration of NaHSO3 produced significant increases in the quantity of blue formazan spots in rice leaves (Figure 2). Only a trace were detected in the untreated control (leaf segments without NaHSO3), but in samples treated with 5 mM NaHS〇3, levels increased to 39.6 % pixels (Figure 3A). Generation of H2O2 in leaf segments was determined by the occurrence of reddish-brown staining (DAB-H2O2 polymerization). In the presence of NaHSO3, rice seedlings responded with rapid generation of H2O2 and the appearance of an intensive reddish-brown stain, particularly with NaHSO3 concentrations ranging from 2 mM to 5 mM (Figures 2 and 3B). By 5 mM NaH-SO3 treatment this reached to one half pixel of the entire area of the leaf segment. Microscopic observations of leaf epidermis indicate that H2O2 was predominantly found in the stomata's guard and subsidiary cells and in the cell wall of partial epidermal cells, while was found primar-ily in the epidermal cells (Figure 2).
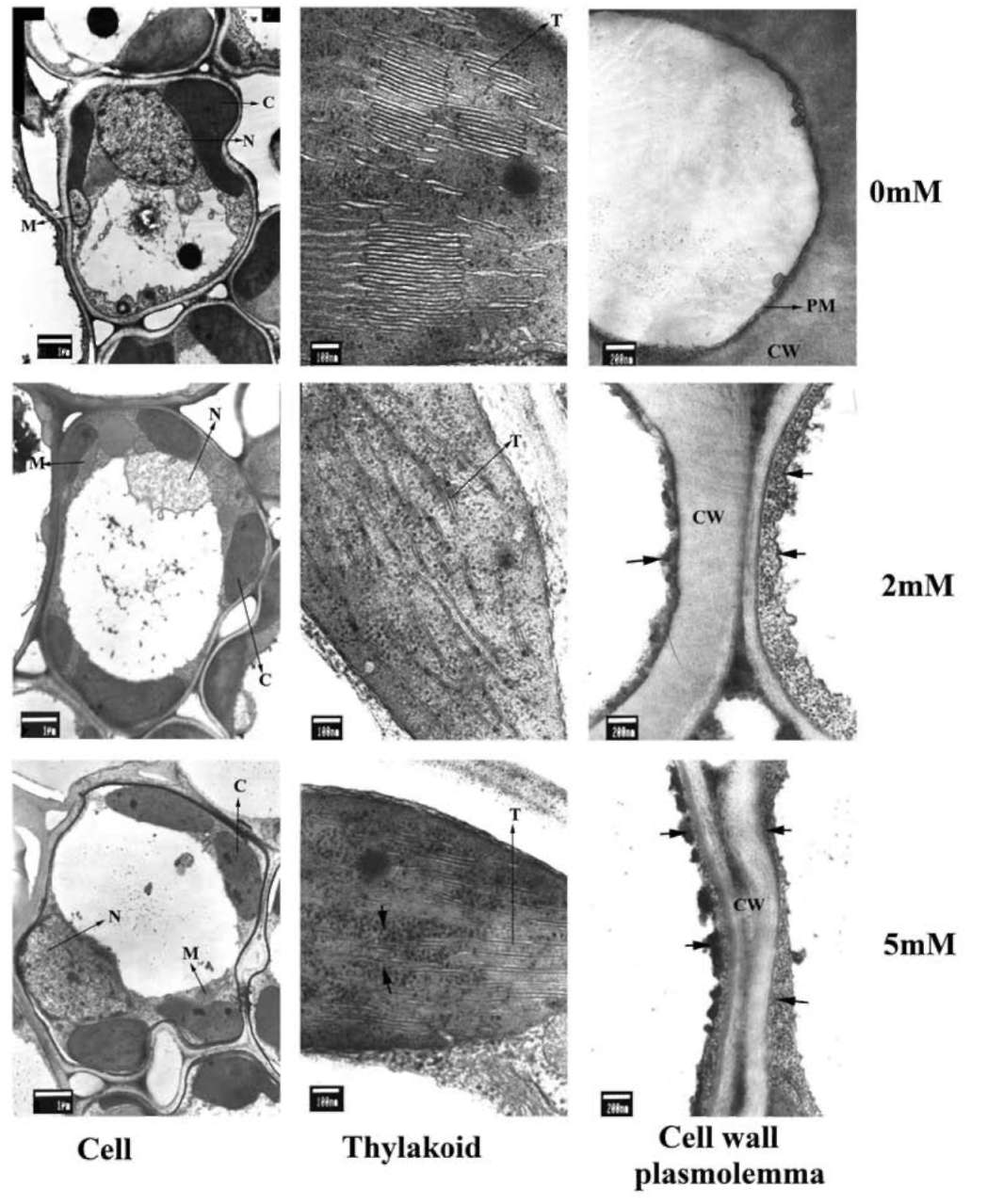
Cytochemical localization of NaHSO3-induced H2O2 generation in rice leaves
Electron-dense precipitates of cerium perhydroxides, produced from the reaction of H2O2 with CeCl3, were not detectable in the cell wall, plasmalemma or chloroplasts of the control seedlings' mesophyll cells. Treatment with NaHSO3 resulted in the intensive formation of stained pre-
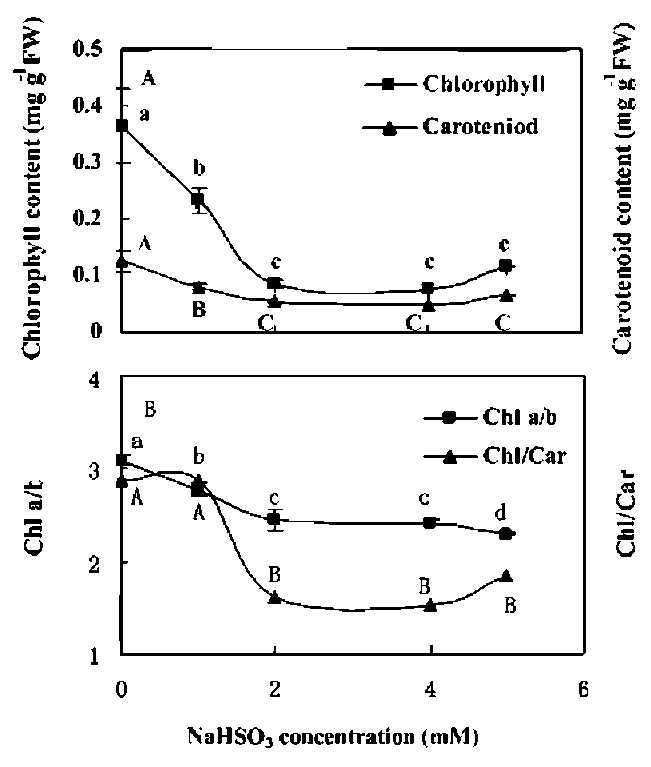
Figure 1. Changes in contents of chlorophyll and carotenoids. (A) ratios of Chl a/b and Chl/Car; (B) in leaves of rice seedlings cultured with sodium bisulfite (NaHS〇3). Rice seedlings were cultured in hydroponics with 1/2 Hoagland solution and various concentration of NaHSOg for 3 d. Mean values with different letters are significantly different atp < 0.05, n=3.
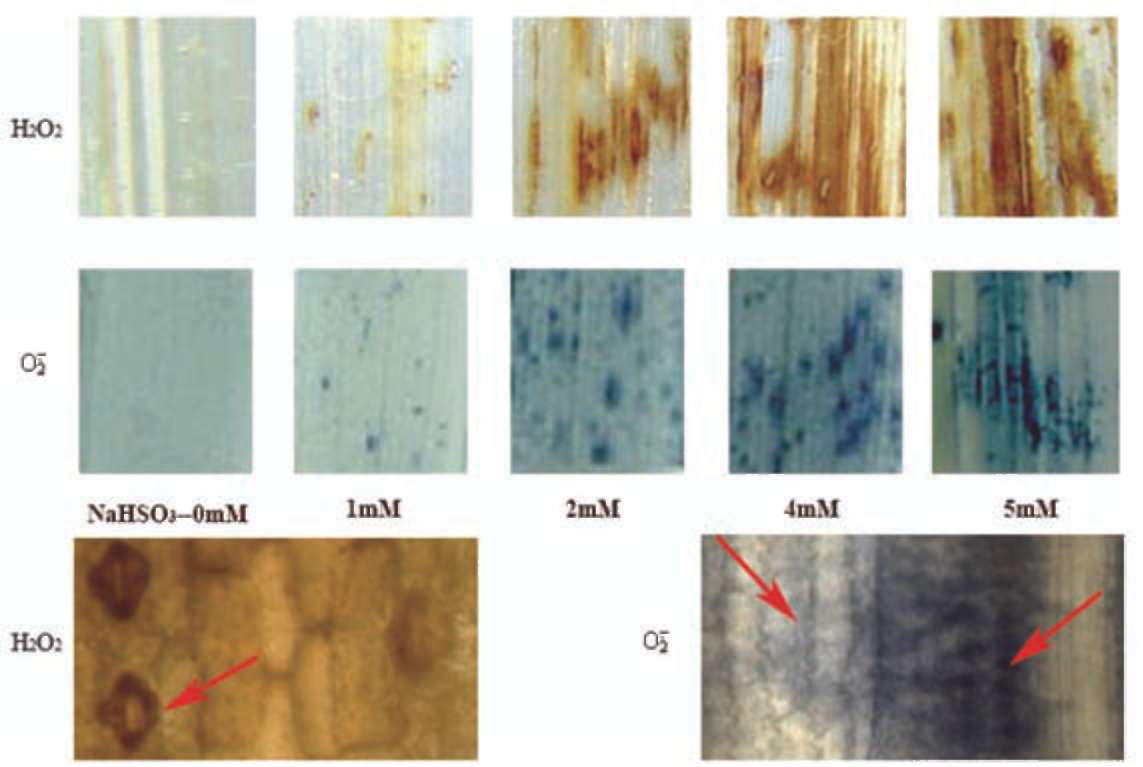
Figure 2. Images of H2O2 (top) and (bottom) generation in leaf tissue induced by NaHS〇3. Reddish-brown spots are H2O2-DAB reaction product, dark-blue formazan spots are -NBT reaction product. The lower side is microscope pictures of tissue localization of H2O2 and generation sites in leaf epidermis, which show presence of H2O2 in guard and subsidiary cells of stomata and cell wall of epidermal cells, but is found in most epidermal cells. See red arrows in figure.