184
Botanical Studies, Vol. 52, 2011
sensitive to the hormone. Such plants include: rose (Batista et al., 2009; Macnish et al., 2010), Christmas cactus (Serek and Reid, 1993), bougainvillea (Chang and Chen, 2001), geranium (Cameron and Reid, 2001), hibiscus (Reid et al., 2002), and kalanchoe (Zeddy et al., 2003). Ethylene trans-port can thus be bi-directional in regulating the flowering of some plants.
For several species of azalea, shoots older than 15 weeks respond to temperature, photoperiod, and Cycocel treatments (Shanks and Link, 1968) with a higher flower density than those of younger shoots. Mango shoots 8 weeks and older are more responsive to lower temperatures in forming flower buds than 2 or 4 week-old shoots are (Nunez-Elisea and Davenport, 1995). According to Ramina et al. (1979), the dry/fresh weight ratio and the content of soluble substances of bougainvillea increase with an increasing leaf area and with a larger number of developing inflorescences. This observation reveals that an increased assimilate accumulation in the reproductive shoots enhances flowering in bougainvillea, and indicates that the branch growth of woody plants must reach a certain maturity before the flower bud forms.
The bougainvillea inflorescence originates from a determinate shoot adjacent to an indeterminate vegetative axillary bud at each node (Figure 1). A thorn-inflorescence refers to a typical thorn containing a developing axis. The fully developed axis is defined as inflorescence hereafter (Hackett and Sachs, 1967). Although bougainvillea has axillary buds accompanying the growth of shoots, the shoot with accelerated growth is non-flowering. Abortion of the inflorescences at various stages of shoot develop-ment is a conspicuous phenomenon in bougainvillea. It was previously mentioned that the bending of bougainvil-lea shoots increases the flowering of buds, the blossom size of flowers and the endogenous ACC content. Shoot bending also resulted in greater ethylene production, causing earlier maturation and flowering. Ethylene may be an important factor in the flowering of bougainvillea (Liu and Chang, 2011). However, which shoot developmental stages of ethylene-sensitive are the least studied. The aim of this research, then, is to elucidate how shoot developmental stages and ethephon affect the flower formation of bougainvillea. The results will provide a basis for future research on the control of flowering in bougainvillea.
MATERIALS AND METHODS
Plant material
One-year-old seedlings of potted 'Taipei Red' bougain-villea were obtained from a commercial bougainvillea nursery and planted in 7-inch pots filled with 2,900 mL medium, composed of field soil and peat in a ratio of 7:3. The seedlings were on average 54.2 cm tall with a mean of 16.2 nodes. During the experimental period, the plants were thoroughly watered every 1 to 3 days, when the surface of the medium appeared slightly dry. Each pot was fertilized monthly with 3 g of 14-14-14 Osmocote.
Effect of ethephon on shoot developmental stages
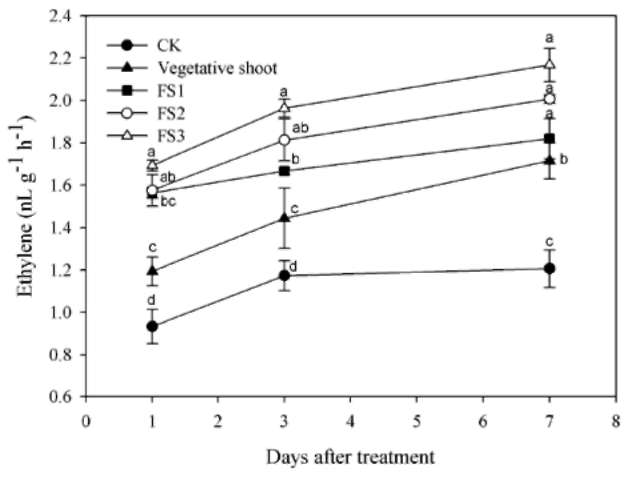
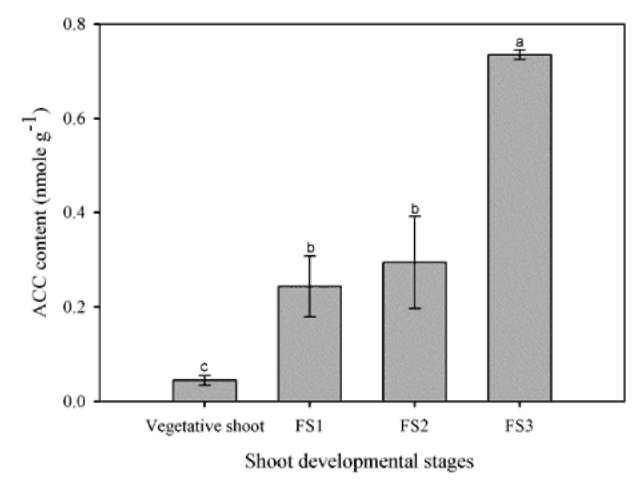
The shoots were first categorized into several classes (Figure 1). Vegetative shoots that exhibited strong growth with prolific and dense leaves. Their florets did not develop fully on the thorn-inflorescence axis; peduncles became thorns. Flowering shoot stages (FS1 to FS3) were characterized as follows: FS1) fully developed thorn-inflorescence axes and florets with elongated peduncles that remained soft and with a tendency towards reproductive growth; FS2) visible flower buds and well-developed, FS3) flower buds in full bloom on the blooming shoot. Plants at each of these four described shoot developmental stages were sprayed with 75 mg L-1 ethephon (2-chloro-ethylphosphonic acid). Ethephon was sprayed once, 50 mL per each pot. After treatment, the plants were grown in a greenhouse where the shoot developmental stage-specific effect of ethephon on flower formation, flower drop and leaf drop could be observed. Each developmental stage was represented by five potted plants. The control plants were at the vegetative shoot stage and received no ethep-hon treatment.
1-Aminocyclopropene-1-carboxylate (ACC) content
ACC measurements were determined from 1 cm in-ternodes segments of the tested plants (cut from 10 cm below the apical bud). The method developed by Lizada and Yang (1979) was modified to perform the analyses. All samples were washed in test tubes with 5 mL of 80% EtOH for subsequent extraction in a hot water bath at 70°C for 20 minutes, followed by centrifuging. This procedure was repeated twice, and the mixture of the two extracts was further concentrated in a vacuum until it became anhydrous. Distilled water was then added to the concentrated sample to a final volume of 1 mL. The prepared
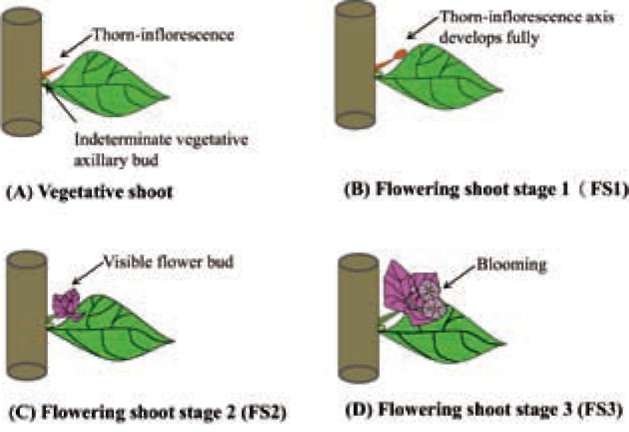
Figure 1. Sketch map of different stages of shoot development in bougainvillea (A) Florets on thorn-inflorescence axis do not fully develop, and peduncle becomes a thorn (B) Thorn-inflorescence axis fully develops, peduncle elongates and remains soft (C) Flowering shoot with visible flower bud (D) Flowering shoot with fully blooming shoot.