LIU et al. ― Low light affecting TIAs and genes
193
RESULTS
Spectra and radiation of the plastic film
The plastic film induced a low light/UV light environment compared to the fluorescent light (300 nm-1,100 nm). As shown in Table 1, UV radiation under plastic film only reached 6% of that under fluorescent light, and the total radiation under fluorescent light was higher (8.31 W m-2) than that under plastic film (0.769 W m-2).
Alkaloid content analysis under low light
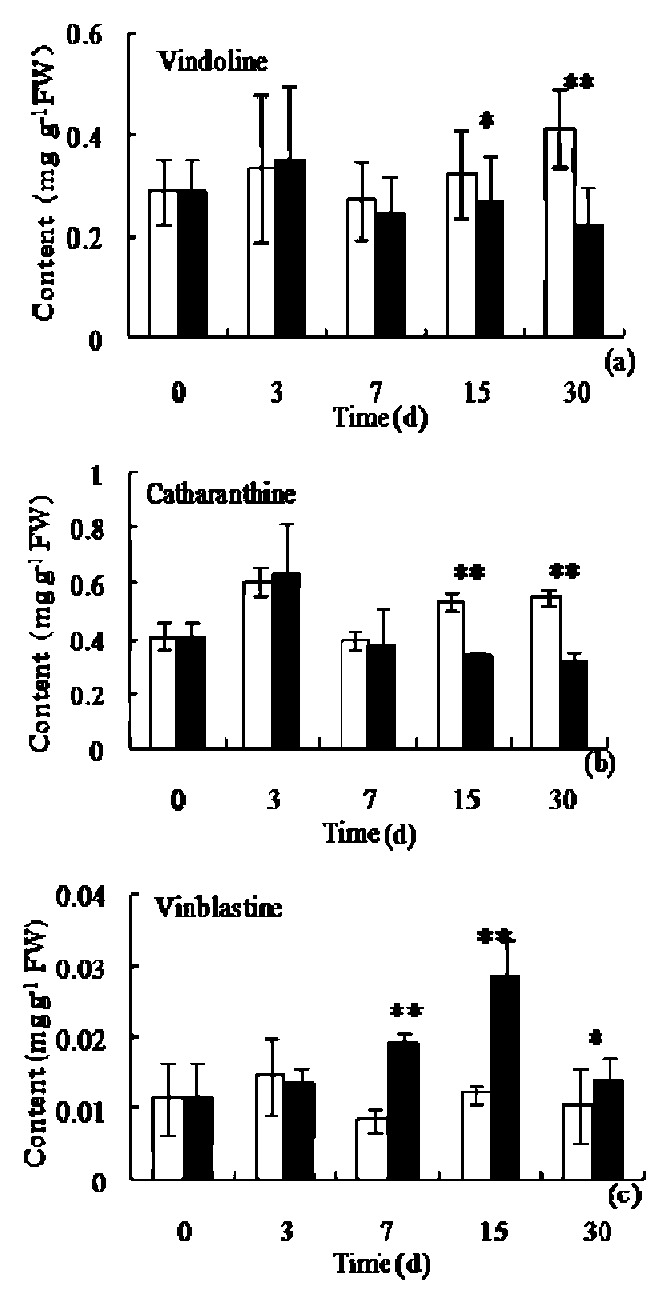
The amount of VIN and CAT present in leaves initially showed a slight increase, reaching its peak (0.32 ±0.07 mg g-1 FW and 0.63 ±0.18 mg g-1 FW) on the 3rd day of the treatment. This was followed by a significant decrease (p<0.05) over 7 days of treatment, (Figure 1a and b). The change of dimeric alkaloid VBL content in treatment showed significant difference from that under fluorescent light (p<0.05), the VBL content became higher than that of control, with its peak value around 0.028 ±0.0051 mg g-1 FW on the 15th day (Figure 1c).
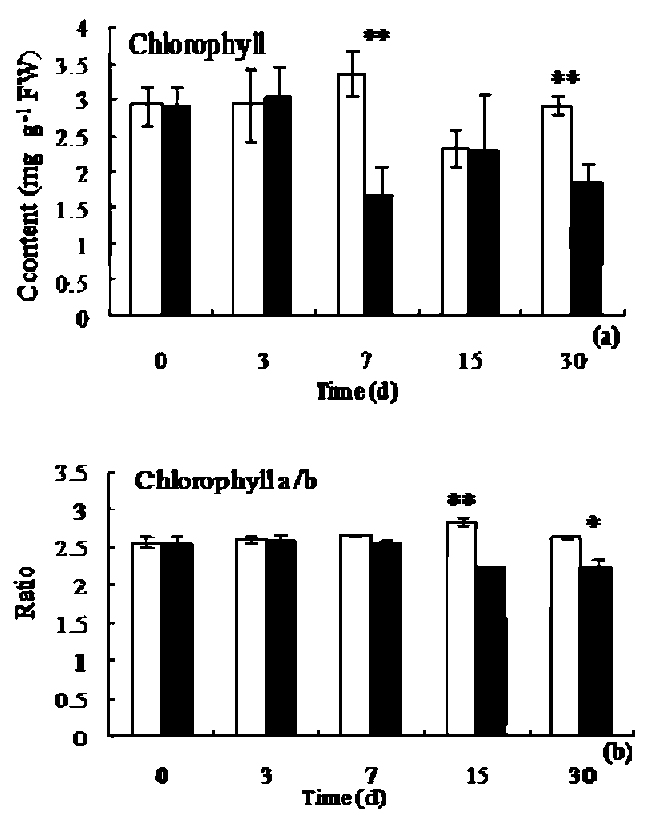
Chlorophyll content analysis under low light
The total chlorophyll content in control leaves gradually increased on the first 7 days and then decreased, whereas plastic film treatment resulted in a little increase of the chlorophyll content on the first 3rd day, and significant decrease (p<0.05) after 3 day of treatment, the lowest value (1.71±0. 35 mg g-1 FW) was recorded on the 7th day (Figure 2a). The ratio of chlorophyll a and chlorophyll b in treatment leaves was consistently lower than that under fluorescent light (Figure 2b).
Gene expression analysis under low light
The mRNA levels of Tdc, Str, D4h and Dat were mea-
sured in leaves under fluorescent light and plastic film, As shown in Table 2, significant differences in gene expression were observed between the treatment and the control. There were significant increases in Tdc mRNA levels between 3 h and 6 h during shading treatment. Str expression was significantly up-regulated at 72 h during shading culture. Furthermore, the transcription levels of D4h and Dat were consistently higher in the treatment than in the control, and mRNA levels of D4h and Dat were signifi-
Figure 1. Effect of plastic film on the amount of vindoline (VIN), catharanthine (CAT) and vinblastine (VBL) in the leaves of aseptic C. roseus seedlings. Data shown are the mean and standard deviation (*p<0.05, **p<0.01; □, control;國,red film).
Table 1. Spectra and radiant energy of control and the plastic film (W m-2).
|
Light Spectra
|
Ultraviolet 300-400 nm
|
Blue
400-510 nm
|
Green
510-610 nm
|
Red
610-720 nm
|
Near infrared
720-1,100 nm
|
Total radiation 300-1,100 nm
|
||
The plastic film
|
0.0173b (6.0)
|
0.0483b (1.66)
|
0.101b (2.97)
|
0.432b (39.6)
|
0.203b (34.1)
|
0.769b (9.3)
|
||
|
Control
|
0.288a
|
2.914a
|
3.405a
|
1.106a
|
0.596a
|
8.31a
|
||
Values in parenthesis are the percents of radiant energy of different light wave bands in overall radiant energy. Means within a column followed by the same letters are not significantly different by SPSS analysis p<0.05. W m-2 is light energy irradiation per meter square.