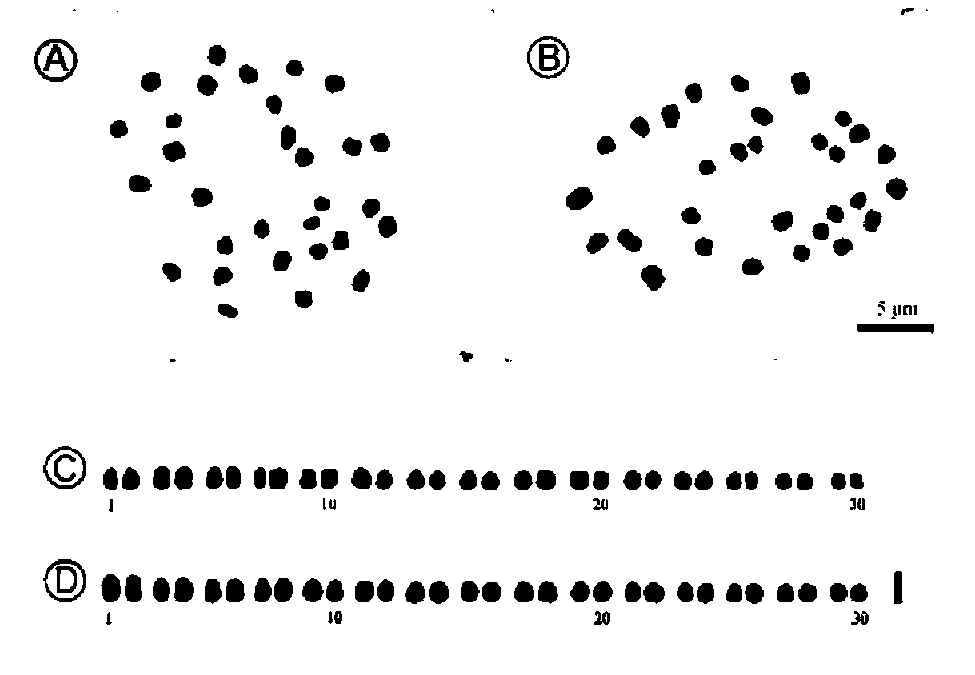
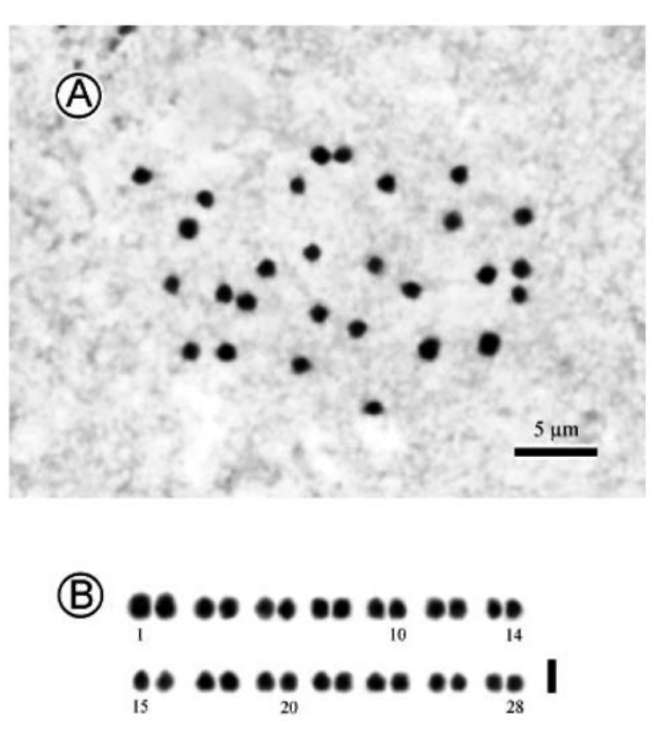
Science Council, Taiwan; and the Royal Society of Edinburgh.
LITERATURE CITED
Blanc, P. 2003. S'eviter, se reajuster, se mimer, se repeter - de l'art de la cohabitation chez les plantes de sous-bois. Hom-mes et Plantes 48: 22-37.
Boghdan, K.S. and F.A. Barkely. 1972. Stomatal patterns in the genus Begonia. Phytologia 23(4): 327-333.
Gan, Y., L. Zhou, Z.J. Shen, Z.X. Shen,Y.Q. Zhang, and G.X. Wang. 2010. Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot. Stud. 51: 325-336.
Hoover, W.S. 1986. Stomata and stomatal clusters in Begonia: ecological response in two Mexican species. Biotropica
18(1): 16-21.
Hughes, M. and C. Coyle. 2009a. Begonia section Petermannia on Palawan (Philippines), including two new species. Edinburgh J. Bot. 66(2): 205-211.
Hughes, M., D. Girmansyah, W.H. Ardi, and Nurainas. 2009b. Seven new Begonia species from Sumatra. Gard. Bull. Sing.
61(1): 29-44.
Hughes, M., C. Coyle, and R. Rubite. 2010. A revision of Begonia section Diploclinium on the Philippine island of Palawan, including five new species. Edinburgh J. Bot. 67(1): 123-140.
Hughes, M. and A.G. Miller. 2002. A new endemic species of Begonia (Begoniaceae) from the Socotra Archipelago. Edinburgh J. Bot. 59(2): 273-281.
Kokubugata, G. and D.A. Madulid. 2000. Chromosomal study of four plant-taxa in Batan Island, the Philippines and the Yaeyama Group, Ryukyu Islands, Japan. Natl. Sci. Mus.
Monogr. 18: 139-144.
Levan, A., K. Fredga, and A.A. Sandberg. 1964. Nomenclature for centromeric position on chromosome. Hereditas 52:
201-220.
Peng, C.-I, Y. Liu, S.M. Ku, Y. Kono, and K.F. Chung. 2010.
Begonia xbreviscapa (Begoniaceae), a new intersectional natural hybrid from limestone areas in Guangxi, China. Bot.
Stud. 51: 107-117.
Rubite, R.R. and D.A. Madulid. 2009. The rediscovery of Philippine Begonias. Blumea 54: 267-268.
Tebbit, M.C. 2005. Begonias. Cultivation, Identification and Natural History. Timber Press, Oregon, USA.