HUANG et al. — Defensin with angiotensin converting enzyme inhibitory activity
259
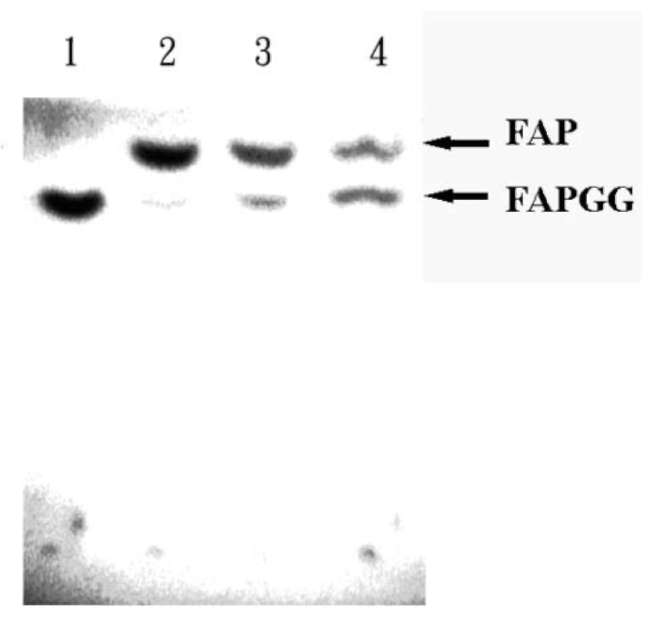
ture for 10 min. Then 800 fL of methanol was added to stop the reaction. The blank experiment contained FAPGG only; in the control experiment, ACE reacted with FAPGG under the same conditions. Each was dried under reduced pressure and redissolved with 400 fL of methanol, and 50 fL was spotted on a silica gel 60 F254. The FAPGG and FAP (ACE hydrolyzed product) were separated by TLC in 1-butanol-acetic acid-water, 4:1:1 (V/V/V), and observed under UV light.
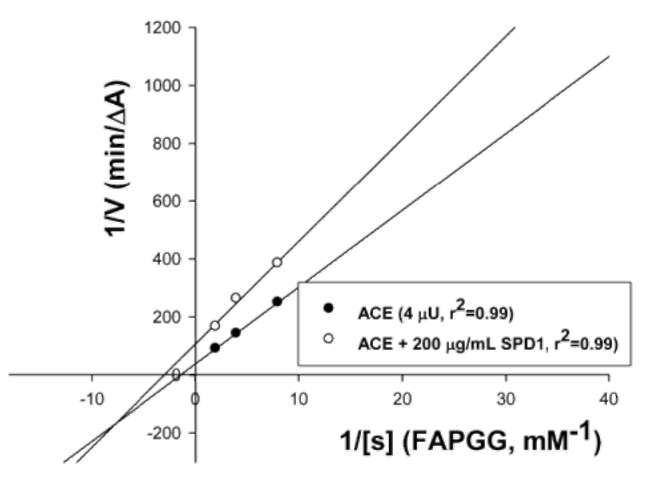
Kinetic properties of ACE inhibition determination by defensin
The kinetic properties of ACE (4 fU) without or with SPD1 (200 (g/mL) in a total volume of 250 [L were determined using different concentrations of FAPGG as substrate (0.1 mM to 0.5 mM). The Km (without SPD1) and Km' (with SPD1) were calculated from Lineweaver-Burk plots, where Km' was the Michaelis constant in the presence of 200 (g/mL SPD1.
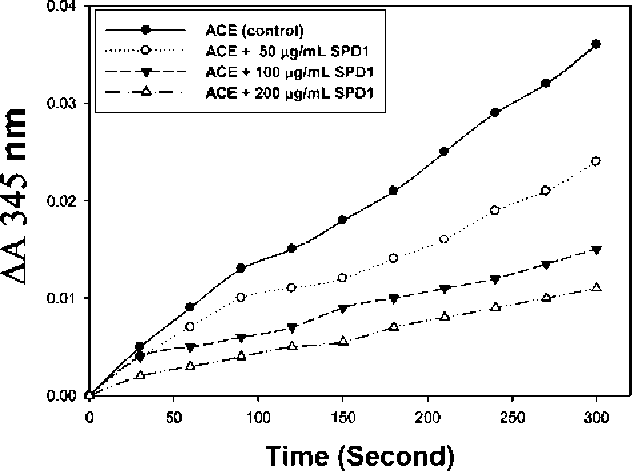
Figure 1. Inhibitory activity of different amounts of defensin (SPD1) (0, 50, 100 and 200 (ig/mL) from sweet potato storage root on ACE activity (AA 345 nm).
ACE inhibitory activity determination by de-fensin trypsin hydrolysates
affinity chromatography, which yielded highly purified His-tagged SPD1. Preparing SDS-PAGE (Huang et al., 2008a) was used as the next step for SPD1 purification.
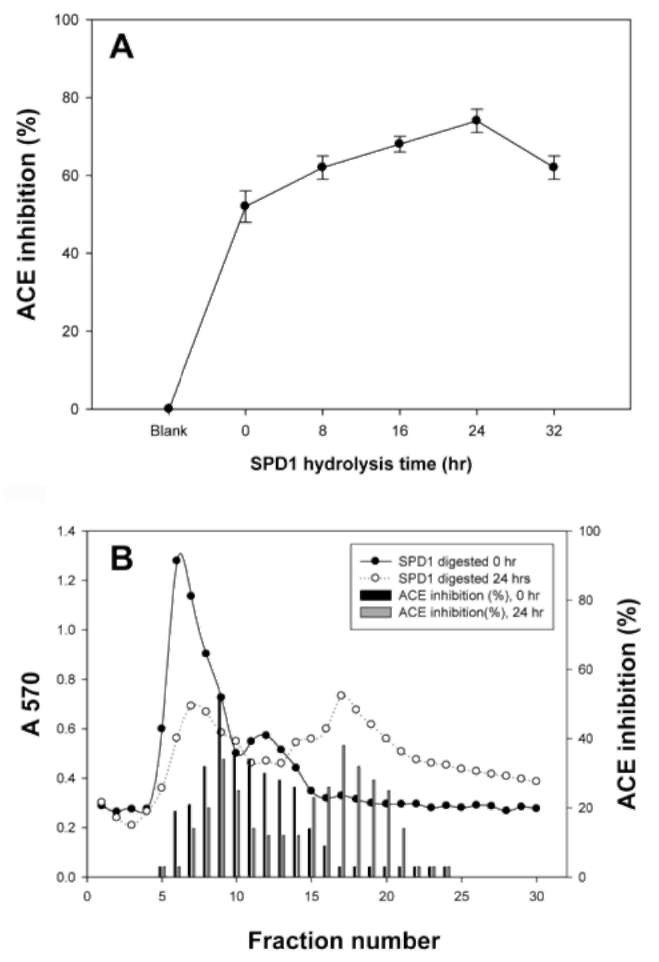
Six mg of SPD1 were dissolved in 1 mL of 50 mM Tris-HCl buffer (pH 7.9). Then 0.1 mL of 12 mg of trypsin was added and hydrolysis was carried out at 37°C for 8, 16, 24 and 32 h. After hydrolysis the solution was heated at 100°C for 5 min to stop enzyme reaction. The trypsin was heated before SPD1 hydrolysis for the 0 h control reaction. Each of the 60 [iL SPD1 hydrolysates was used in spectro-photometry to determine ACE inhibition.
Spectrophotometric determination of ACE inhibitor activity of defensin
Purified SPD1 was used to determine ACE inhibitory activity. Figure 1 shows time progression for the effect
of the different amounts of SPD1 (0, 50, 100 and 200 (g/
mL) on the ACE activity (AA 345 nm). Compared with the ACE only (control), it was found that the higher the amount of SPD1 added during a 300 sec reaction period, the lower the AA 345 nm would be. Figure 1 shows that purified SPD1 could inhibit ACE activity in a dose-dependent manner.
Defensin tryptic hydrolysate chromatograms on a Sephadex G-50 column
The unhydrolyzed SPD1 and tryptic SPD1 hydrolysates at 24 h were separated by Sephadex G-50 chromatography (1 x 60 cm). The column was eluted with 20 mM Tris-HCl buffer (pH 7.9). The flow rate was 30 mL/h, and each fraction contained 2 mL, the absorbance of which was determined at 570 nm.
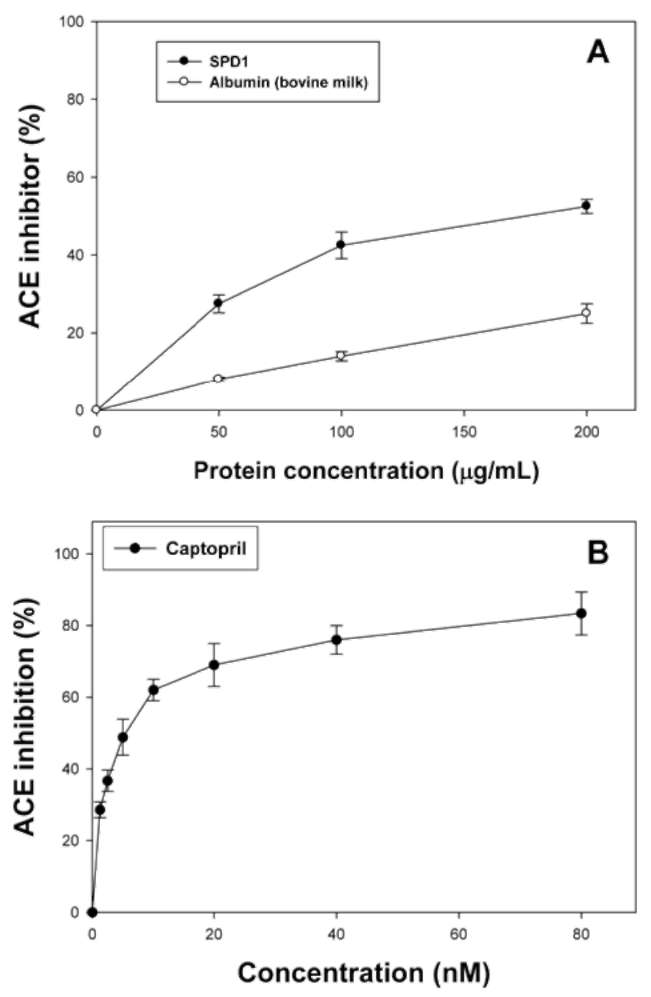
Effects of defensin, BSA and captopril on ACE activity shown by spectrophotometry
We were interested in learning whether BSA also exhibited ACE inhibitory activity. Figure 2A shows the effects of SPD1 (0, 50, 100, and 200 ig/mL), BSA (0, 50, 100, and 200 (ig/mL) or Captopril (Figure 2B; 0, 1.25, 2.5, 5,
10, 20, 40 and 80 nM; corresponding to 0, 108.5, 217, 434, 868, 1,736, 3,472 and 6,844 ng/mL, respectively) on ACE
activity. BSA showed less ACE inhibitory activity (less than 25% inhibition) without dose-dependent inhibition patterns. However, SPD1 exhibited dose-dependent ACE inhibitory activity (50~200 (ig/mL giving, respectively, 27.56 ~ 52.58% inhibition). From calculations, the 50%
inhibition (IC50) of SPD1 against ACE activity was 190.47
(g/mL compared to that of 10 nM (868 ng/mL) for Cap-topril, which was similar to the report (7 nM) by Pihlanto-Leppala et al. (1998); while the IC50 of yam dioscorin was 250 (ig/mL (Hsu et al., 2002). Both BSA and purified SPD1 were proteins, but only the purified SPD1 showed specific dose-dependent ACE inhibitory activity. In the
Statistical analysis
Means of triplicates were calculated. Student's t test was used for comparison between two treatments. A difference was considered to be statistically significant when p < 0.05.
RESULTS and DISCUSSION
Expression of defensin in E. coli
SDS-PAGE analysis of SPD1 crude extracts from the transformed E. coli (M15) showed high amounts of a poly-peptide with the expected molecular mass (ca. 8,600 Da). This polypeptide was found as a soluble protein in the supernatant, and was absent in protein extracts obtained from E. coli transformed with pQE-30 vector. The expressed protein was purified from crude extracts by Ni2+-chelate