CHEN et al. ― Sweet potato catalase
421
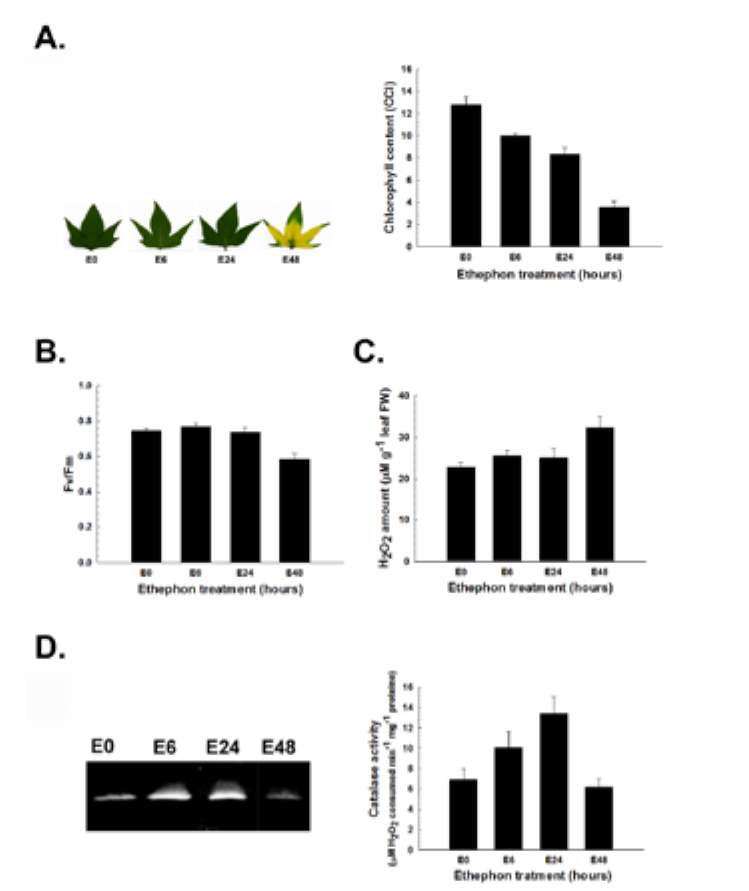
L2 to L3, then increased again from L4 partial and L5 completely yellow senescent leaves, which had an amount close to that of L1 leaves (Figure 2C). Temporal and spatial patterns showed that the sweet potato catalase isoform was specifically expressed in leaves. Its activity was first detected in immature L2 leaves, increased and reached its maximum in mature L3 leaves, gradually decreased in partially yellow senescent L4 leaves until it was almost unde-tectable in completely yellow senescent L5 leaves (Figure 2D). No significant catalase activity was detected in sweet potato stems and roots (Figure 2E). The quantitative cata-lase activity assay matched the qualitative in-gel activity assay (Figure 2D and 2E). These data conclude that sweet potato contains a major leaf-type catalase isoform with maximum activity in mature L3 leaves. The leaf-type
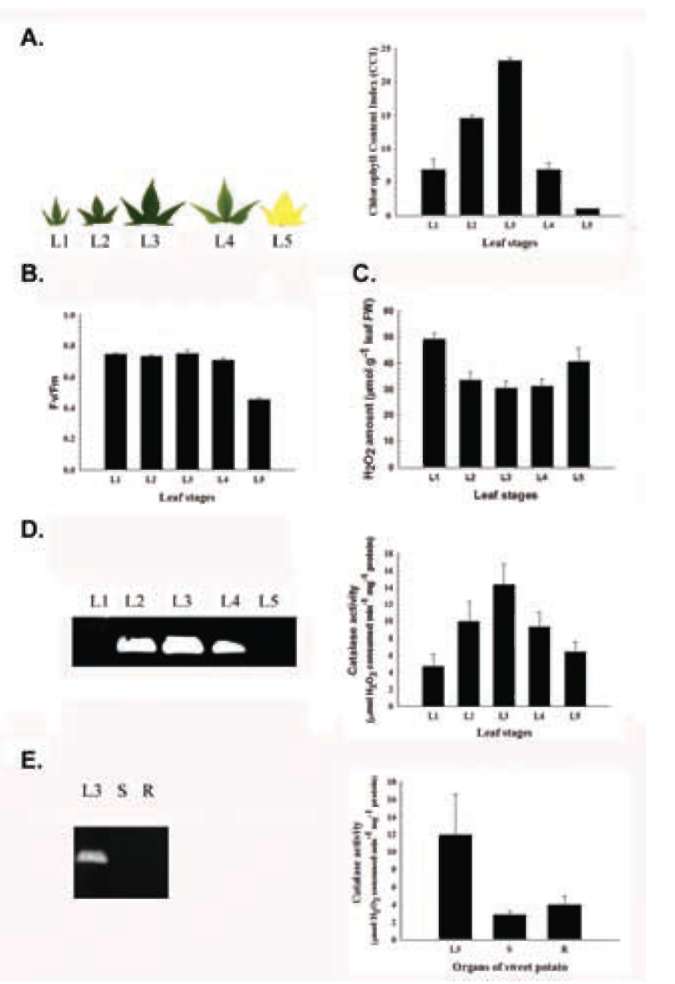
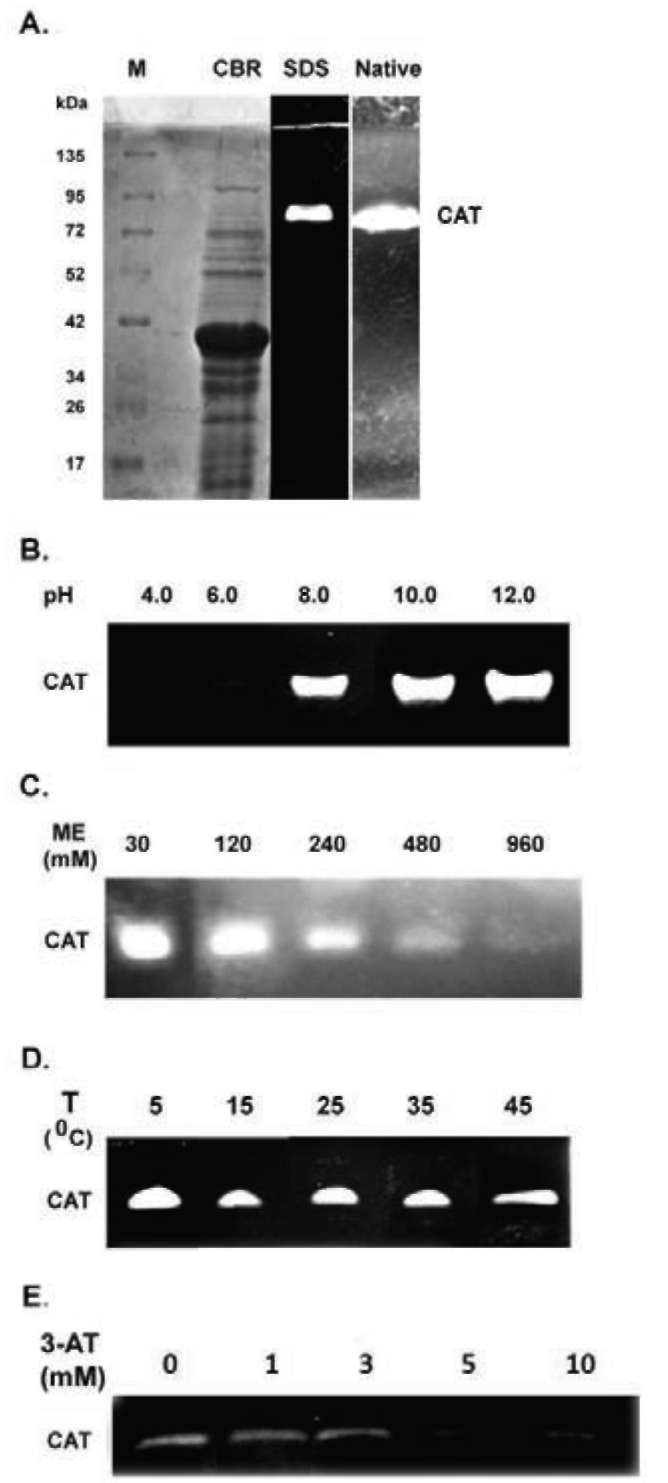
Figure 1. Detection and analysis of catalase activity from sweet potato mature L3 leaves. (A) Detection of catalase activity in native- and SDS-PAGE. CBR, Native and SDS denote Coo-massie Brilliant Blue R250 staining, native- and SDS-PAGE, respectively. M and CAT denote molecular mass and catalase, respectively; (B) Effect of pH on catalase activity; (C) Effect of p-mercaptoethanol on catalase activity; (D) Effect of temperature on catalase activity; (E) Effect of 3-amino-1,2,4-triazole on cata-lase activity. The ME, T, and 3-AT denote p-mercaptoethanol, temperature, and 3-amino-1,2,4-triazole, respectively. The experiments were performed three times and a representative one was shown.
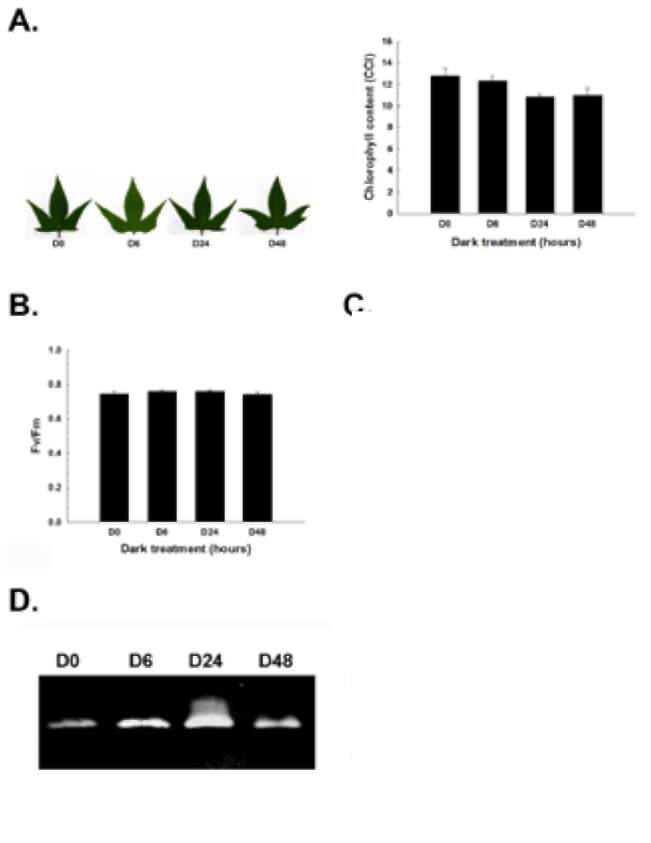
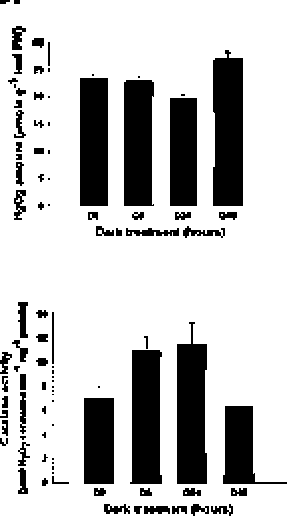
Figure 2. Temporal and spatial catalase expression in sweet potato. (A) Leaf morphology and chlorophyll content; (B) Photochemical Fv/Fm; (C) H2O2 amount; (D) Temporal cata-lase activity assay; (E) Spatial catalase activity assay. The leaf morphology, chlorophyll content, photochemical Fv/Fm, H2O2 amount, and in-gel and spectrophotometric catalase activity were analyzed in different leaf stages (L1, L2, L3, L4 and L5) and organs (mature L3 leaf, stem and root) of sweet potato. Stages of leaves were described in "Materials and Methods". L, S and R denote leaf, stem and root, respectively. The data were shown as mean ± S.E.. The experiments were performed at least three times and a representative one was shown.