48
Botanical Studies, Vol. 53, 2012
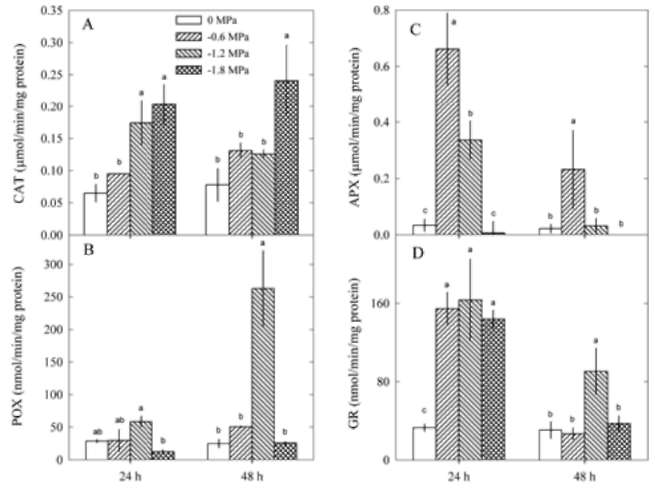
Antioxidant enzyme activities
An analysis of active oxygen-processing enzymes activities
showed that SOD was affected by PEG (P<0.05) and
treatment time (P<0.05). SOD activities were not affected
by PEG after 24 h of treatment, but decreased after 48 h of
treatment (Figure 3). CAT activities were affected by PEG
(P<0.0001) but not by treatment time (P=0.2562). POX,
APX and GR activities were affected by both PEG (2-way
ANOVA, P<0.05) and treatment time (P<0.05). The interaction
of PEG and treatment time on antioxidant enzyme
activities was significant for POX, APX and GR (P<0.05)
but not for CAT (P>0.05).
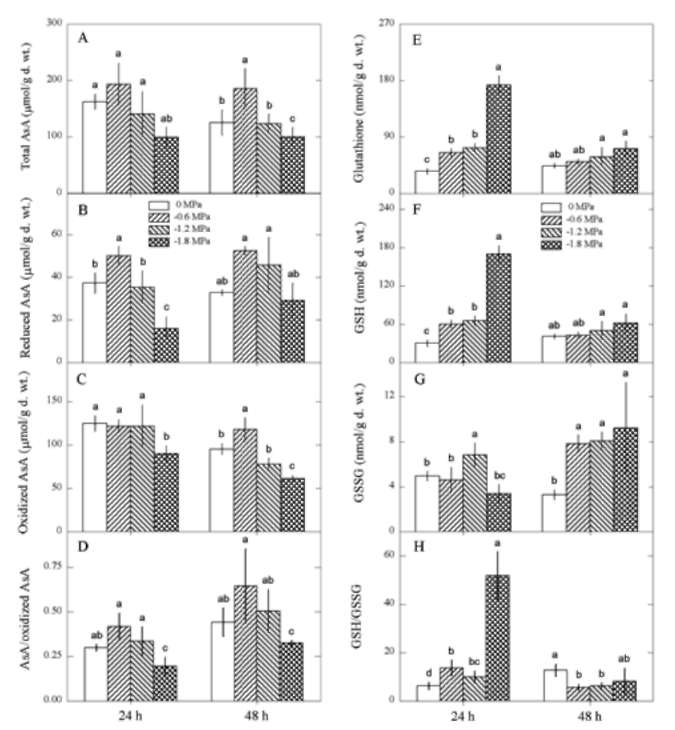
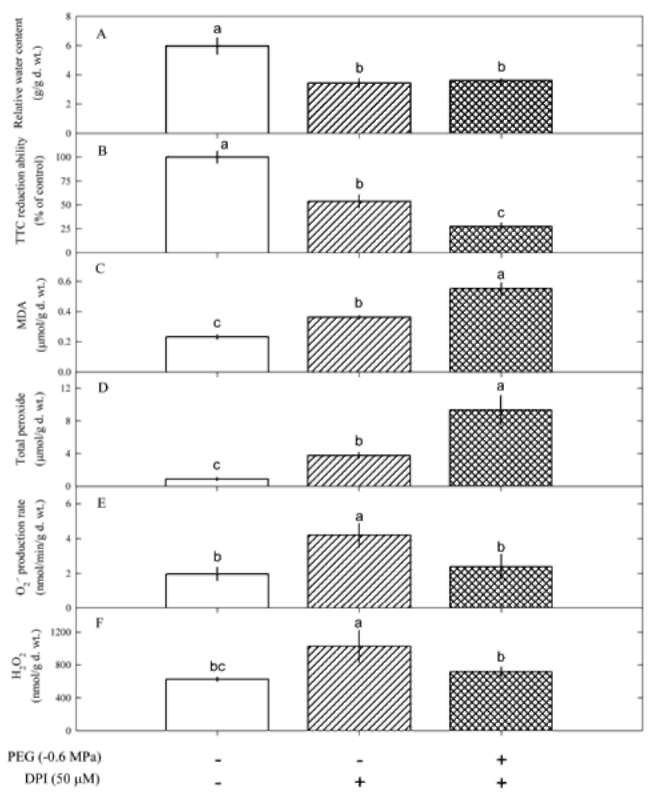
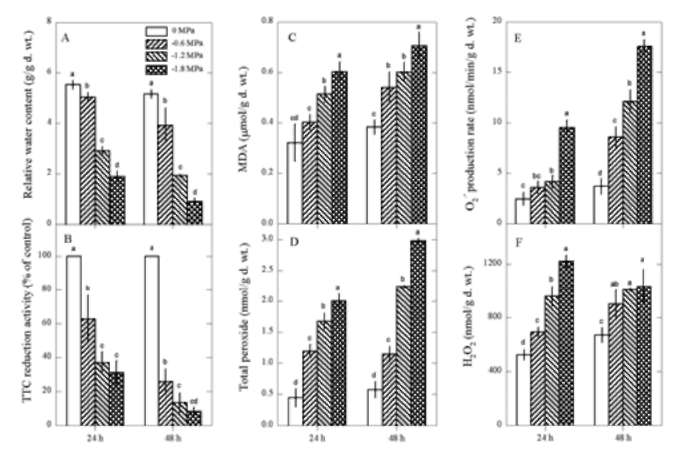
Figure 1. Changes in relative water contents (A), TTC reduction ability (B), MDA contents (C), total peroxide contents (D), O2 - production rate (E) and H2O2 contents (F) in Pluchea indica leaves in response to varying PEG concentrations, i.e. 0, -0.6, -1.2 and -1.8 MPa. Data are present as means士SD (n=3) and different letters indicate significant difference among treatments.
ment time advanced (P<0.05). The interaction of PEG and treatment time on O2 - production rate and total peroxide, MDA and H2O2 contents was significant (P<0.05). These indicate that PEG-induced water deficit can trigger oxida-tive damage in P. indica leaves.
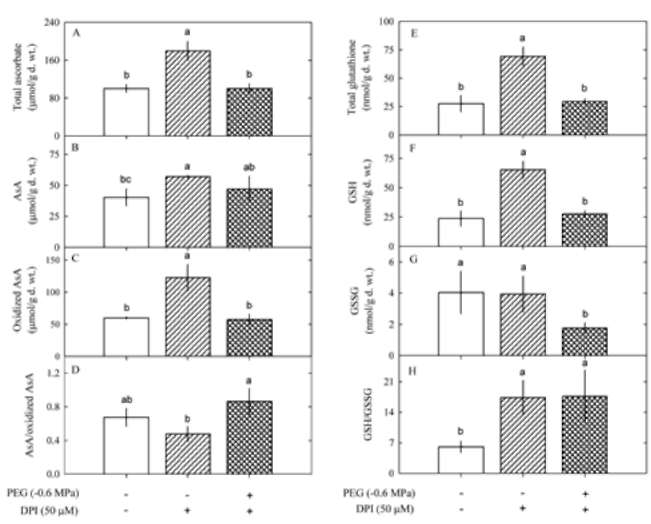
Ascorbate and glutathione contents
We observed changes in antioxidant Ascorbate and
glutathione contents during oxidative damage in P. indica
leaves. PEG (P<0.05) but not treatment time (P>0.05)
affected total AsA and reduced AsA contents while both
PEG (P<0.05) and treatment time (P<0.05) affected oxidized
AsA contents and reduced AsA/oxidized AsA ratios,
the interaction of PEG and treatment time on oxidized AsA
contents and reduced AsA/oxidized AsA ratios was significant
(P>0.05). After exposure to water deficit, total AsA
(Figure 2A) and reduced AsA (Figure 2B) contents and
reduced AsA/oxidized AsA ratios (Figure 2D) increased in
response to -0.6 MPa, but total AsA, reduced AsA and oxidized
AsA contents and reduced AsA/oxidized AsA ratios
decreased in response to -1.8 MPa.
Figure 2. Changes in ascorbate: total AsA (A); reduced AsA
(B); oxidized AsA (C); reduced AsA/oxidized AsA ratio (D),
and glutathione contents: total glutathione (E); GSH (F); GSSG
(G); GSH/GSSG ratio (H) in Pluchea indica leaves in response
to 0, -0.6, -1.2 and -1.8 MPa. Data are present as means±SD
(n=3) and different letters indicate significant difference among
treatments.
Both PEG (P<0.05) and treatment time (P<0.05) affected
total glutathione, GSH and GSSG contents, and the
interaction of PEG and treatment time on total glutathione,
GSH and GSSG contents and GSH/GSSG ratios was significant
(P<0.05). After 24 h of water deficit treatment, total
glutathione (Figure 2E) and GSH (Figure 2F) contents
and GSH/GSSG ratios (Figure 2H) increased as water potential
decreased from 0 to -1.8 MPa, but GSSG contents
only showed a small increase at -1.2 MPa. After 48 h of
treatment, total glutathione (Figure 2E) and GSH (Figure
2F) contents also increased, but their increments were relatively
smaller than at 24 h treatment. The GSSG contents
showed a marked increase after 48 h of water deficit treatment
(Figure 2G), resulting in a drop of GSH/GSSG ratios
(Figure 2H).
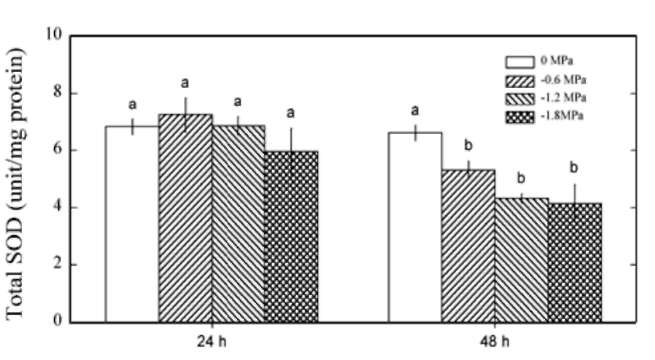
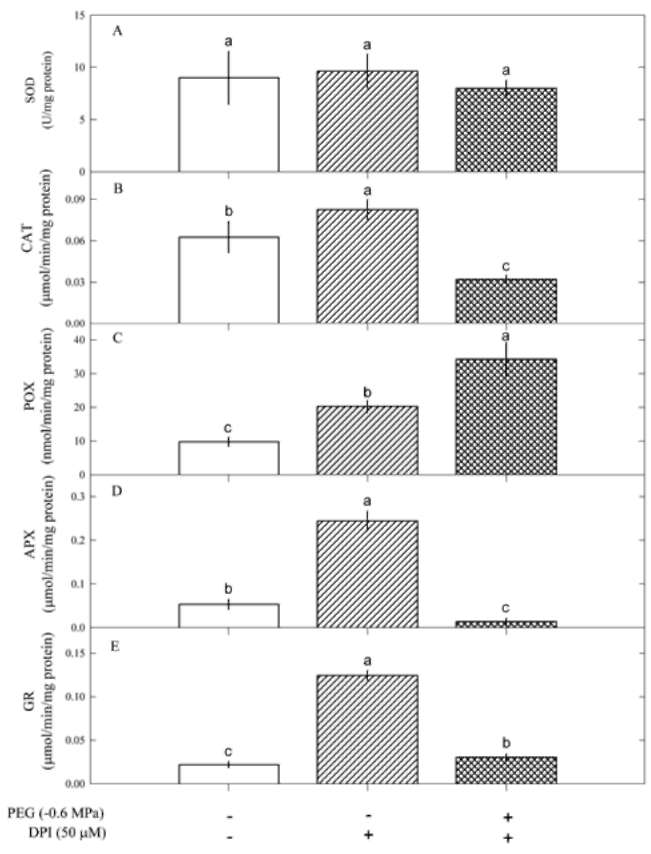
Figure 3. SOD activity in Pluchea indica leaves in response to 0, -0.6, -1.2 and -1.8 MPa. Data are present as means±SD (n=3) and different letters indicate significant difference among treatments.