178
Botanical Studies, Vol. 53, 2012
CONCLUSIONS AND FUTURE PERSPECTIVES.........................................................................................................185
LITERATURE CITED........................................................................................................................................................ 185
INTRODUCTION
Experiments with single-node leaf cuttings from induced potato (Solanum tuberosum L.) plants suggested the possible role of Ca2+ as a mediator of tuberization stimulus (Balamani et al., 1986). A few other studies demonstrated the influence of supplemental Ca2+ on tuberization of potato under field conditions (Ozgen et al., 2000, 2003, 2006; Ozgen and Palta, 2004; Chang et al., 2007) though the exact mechanism of its influence was not studied. Reports also suggested the possible involvement of Ca2+-sensor proteins such as calmodulin (CaM) (Jena et al., 1989) and potato calcium dependent protein kinase (StCDPK1) in tuberization of potato (MacIntosh et al., 1996; Raices et al., 2001, 2003; Ganrgantim et al., 2009). In spite of evidences for the positive role of Ca2+ and various Ca2+-regulated proteins in potato tuberization, the exact molecular mechanism of Ca2+ and Ca2+-induced signal pathways controlling tuberization is not studied well. In this review we endeavor to provide an update on the recent advances and discussion on various aspects of Ca2+-mediated signaling in potato tuberization.
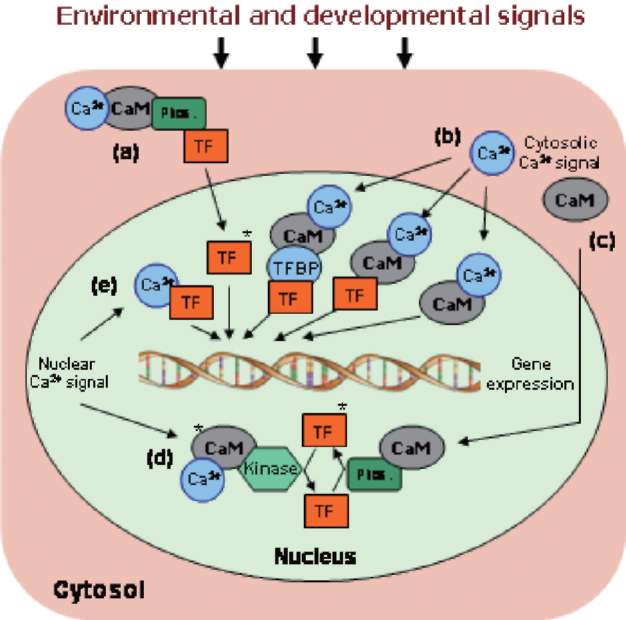
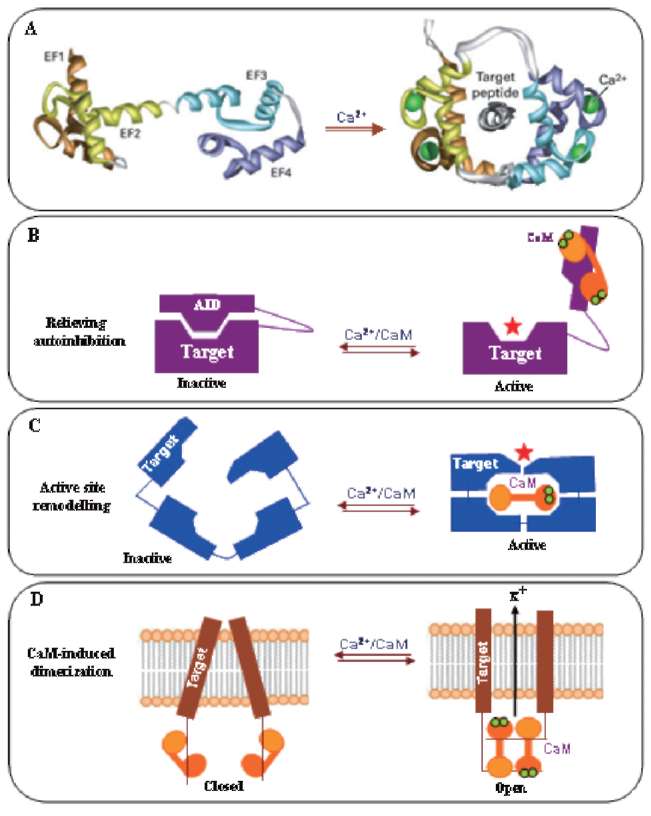
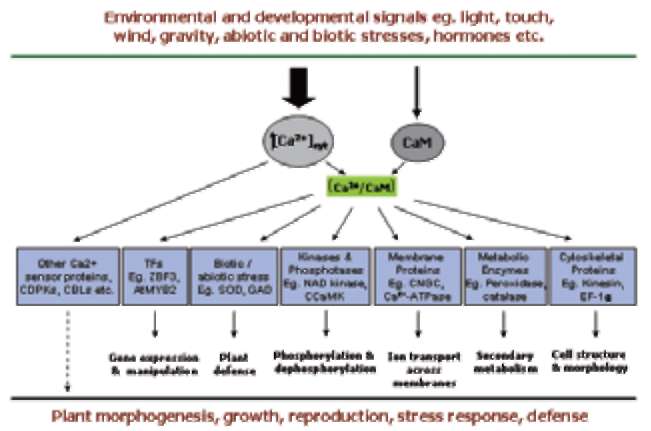
Tuberization is a complex phenomenon involving a morphological transition of an underground shoot to stolon and subsequent tuber formation under complex environmental, nutritional and endogenous regulation (Figure 1). It comprises induction, initiation and growth of the stolon followed by the initiation and growth of the storage organ, tuber. Tuberization in potato serves dual function, as a storage organ and a mean to vegetative propagation. A dynamic change in the expression pattern of metabolic enzymes, endogenous growth regulators, protease inhibitors and accumulation of storage proteins, such as patatins, was observed at the onset of tuberization (Prat et al., 1990; Taylor et al., 1992a, b, 1993; Jackson et al., 1997). A wide variety of soil, environmental and hormonal stimuli are known to be involved in the induction of potato tuberiza-tion (Menzel, 1983, 1985; Vreugdenhil and Struik, 1989; Pelacho et al., 1994). Among the soil factors, calcium (Ca2+) nutrition plays an important role in potato tuberiza-tion. Calcium is an essential component of the plant cell wall giving mechanical strength and providing the medium for normal transport and retention of other elements. It is an essential nutrient and has been shown to affect protein phosphorylation in plants (Budde and Chollet, 1988) through affecting the calcium-binding modulator proteins and protein kinases. Subsequently, protein kinases modulate the activity of many key enzymes through protein phosphorylation, a major mechanism involved in transduc-tion of various Ca2+ signals (Sopory and Munshi, 1998).
NUTRITIONAL AND PHYSIOLOGICAL ROLES OF Ca2+ IN PLANTS
Calcium is a major nutrient required for normal growth and development of plants. As a divalent cation (Ca2+), it has structural roles in the cell wall and cell membranes as a counter cation for anions in the vacuoles (White and Broadley, 2003), and acts as intracellular messenger in the cytosol (Marschner, 1995). The most striking use of Ca2+ ions as a structural element in plants occurs in the marine coccolithophores, which use Ca2+ to form the calcium carbonate plates with which they are covered. In plants, Ca2+ is usually stored as Ca-oxalate crystals in plastids. Calcium is critical for plant cells providing strong structural rigidity by forming cross-links within the pectin polysac-charide matrix (Easterwood, 2002). Calcium deficiency is rare in nature but a few Ca2+ deficiency disorders occur in horticultural crops such as 'tipburn', 'brown heart' in leafy vegetables, 'black heart' in celery, 'blossom end rot' in watermelons, tomato, pepper, 'fruit cracking' in tomato, 'bitter pit' in apples, 'empty pod' in peanuts and 'internal brown spot', 'hollow heart' and 'softrot' in potatoes. With rapid plant growth, the structural integrity of stems and the quality of fruit produced is strongly coupled to Ca2+ availability. Calcium being a universal second messenger acts as a mediator of stimulus-response coupling in the regulation of diverse cellular functions (Allen and Schroeder, 2001). Calcium was also reported to play a role in signal transduction leading to oxidative burst and plant defense (Miura et al., 1999). Calcium is also known to act as an activator of many enzymes like ATPase, phospholipases, amylase and succinate dehydrogenase. Experiments with
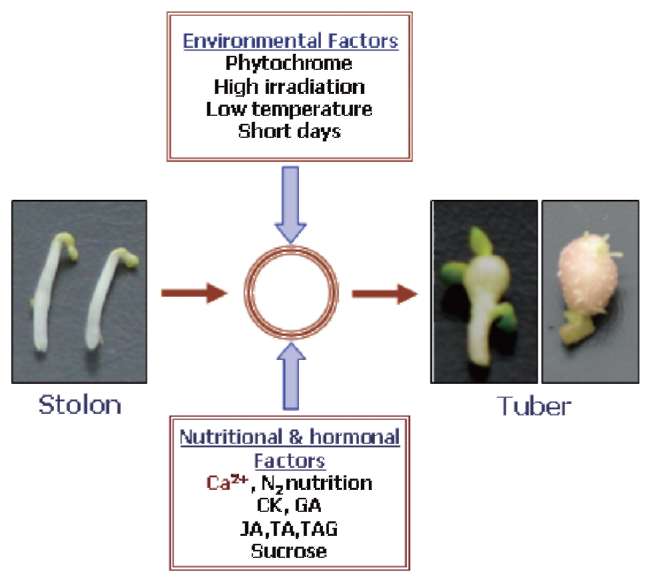
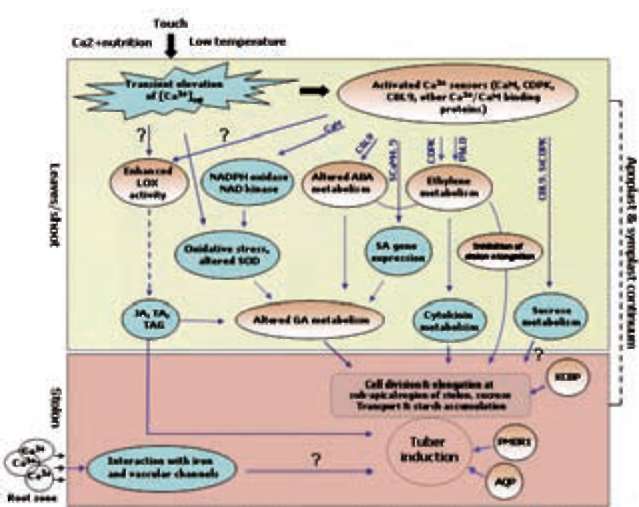
Figure 1. Interaction of various environmental and nutritional factors influencing tuber induction in potato. CaM, calmodulin; CK, cytokinin; GA, gibberellic acid; JA, jasmonic acid; TA, tuberonic acid; TAG, tuberonic acid glucoside.