|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies (2012) 53: 197-206.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecular cloning and functional analysis of bergaptol-O-methyltransferase from Angelica dahurica (Bai Zhi) and using it to efficiently produce bergapten in E. coli
|
|
|
|
|
|
|
|
Shu-Chin LO1,2, Pei-En CHUNG2, and Co-Shine WANG1*
|
|
|
|
|
|
|
|
1Graduate Institute of Biotechnology, National Chung Hsing University, Taichung 40227, Taiwan
2Department of Agricultural Chemistry Division, Taiwan Agricultural Research Institute, Taichung 41362, Taiwan
|
|
|
|
|
|
|
|
(Received September 19, 2011; Accepted February 3, 2012)
|
|
|
|
|
|
|
|
ABSTRACT. Bai Zhi (Angelica dahurica), a Chinese herb, has long been used as a face cream for skin-whitening purposes. One of the known skin-whitening components, 8-hydroxybergapten is a hydroxylated product of bergapten that is converted from bergaptol by bergaptol 5-O-methyltransferase (BMT) in Bai Zhi. The complementary DNA of BMT was cloned from Bai Zhi root using a pair of degenerate primers designed from the highly conserved regions of other plant O-methyltransferases (OMTs). RT-PCR analysis indicated that a single band of DNA fragment corresponding to AdBMT sequence was obtained. The tandem 5'- and 3'-rapid amplification of cDNA ends via polymerase chain reaction was used to obtain the full-length cDNA sequences. The AdBMT cDNA contains an open reading frame of 1,080 bp encoding a BMT polypeptide of 359 amino acids with a calculated molecular mass of 39 kDa and a calculated pi of 5.9. Sequence alignment revealed the considerable sequence similarity of AdBMT to those of other plant OMTs. The AdBMT sequence contains conserved region I-V, similar to other plant OMTs. His-tagged AdBMT was expressed in E. coli and partially purified by ammonium sulfate precipitation. The recombinant AdBMT is most active in potassium phosphate buffer at pH 7.5 and 35°C. The enzyme does not require a divalent cation for activity and the addition of Cu2+, Ni2+, and Co2+ at concentrations even as low as 0.1 mM severely inhibits enzyme activity. A simple and efficient production of bergapten in the E. coli culture overexpressing AdBMT was performed. The bergapten yield is approximately 13-fold higher than that produced by enzymes in the ammonium sulfate-puri-fied fraction. With the supply of bergaptol in the medium, E. coli cells can be used as a potential bioreactor to produce bergapten.
|
|
|
|
|
|
|
|
Keywords: Bai Zhi; Bergapten; Bergaptol 5-O-methyltransferase; cDNA cloning; Enzyme activity.
|
|
|
|
|
|
|
|
Abbreviations: BMT, bergaptol 5-O-methyltransferase; cDNA, complementary DNA; EDTA, ethylenedi-aminetetraacetic acid; IPTG, isopropyl p-D-thiogalactopyranoside; NTA, nitrilotriacetic acid; OMT, O-meth-yltransferases; PAGE, polyacrylamide gel electrophoresis; RT-PCR, reverse transcriptase-polymerase chain reaction; RACE, rapid amplification of cDNA ends; SAM, S-adenosyl-L-methionine; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; UPLC, ultra performance liquid chromatography.
|
|
|
|
|
|
|
|
|
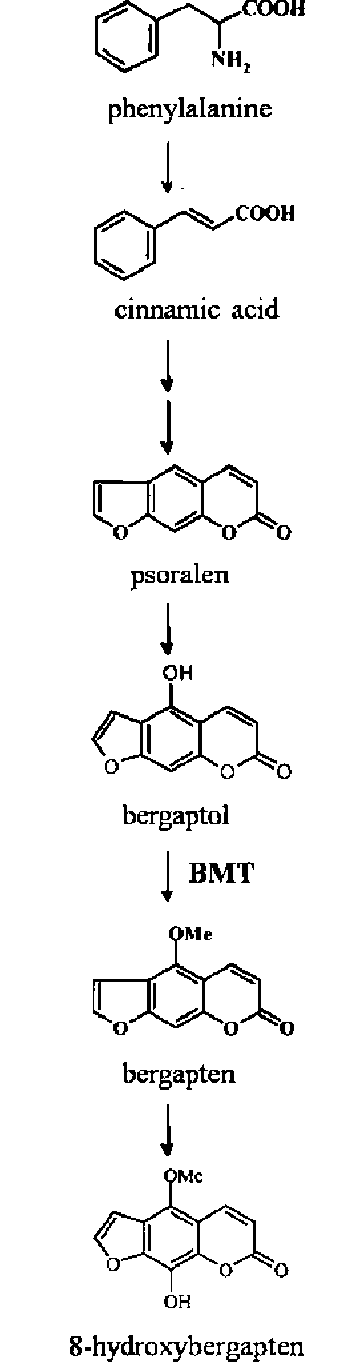
bergapten, xanthotoxin and isopimpinellin, all of which are bioactive compounds. The biosynthetic pathway of these bioactive furanocoumarins was outlined by Hehmann et al. (2004) although the sequence of hydroxylations and O-methylations of psoralen leading to isopimpinellin has not been established. Of those bioactive compounds, bergapten is produced from bergaptol by bergaptol 5-O-methyl-transferase (BMT, Figure 1) and the corresponding OMTs were identified from elicitor-treated Ammi majus cells (Hehmann et al., 2004) and more recently, from Glehnia littoralis cell cultures (Ishikawa et al., 2009). Since BMT is constitutively expressed in G. littoralis cell cultures, it is possible that cell cultures can be used as a potential bioreactor to produce bergapten by supply of psolaren. Bergapten is further converted into 8-hydroxybergapten
|
|
|
|
Methylation by S-adenosyl-L-methionine (SAM) dependent O-methyltransferases (OMTs) is a common modification in natural product biosynthesis. In plants,O-methylation is also required for linear furanocoumarin biosynthesis. Furanocoumarins are synthesized from the phenylpropanoid pathway as phytoalexins or defense-related substances in response to microbial infection (Tietjen et al., 1983; Kuete et al., 2007; Alexander et al., 2008). The most abundant linear furanocoumarins are psoralen,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
that may inhibit mushroom tyrosinase. The inhibition of mushroom tyrosinase by 8-hydroxybergapten was reported to be superior to kojic acid and arbutin, two tyrosinase inhibitors widely used as skin-whitening agents in cosmetics (Piao et al., 2004).
Plant OMTs can be categorized into two major groups (Joshi and Chiang, 1998). Group I includes a class of OMTs with low molecular masses (23 to 27 kDa) and this group of OMTs is Mg2+-dependent. The Group II consists of higher molecular mass OMTs (38 to 43 kDa) that do not require Mg2+ for catalytic activity. Prominent Group II members include caffeic acid, flavonoid, coumarin, and
|
alkaloid OMTs (Frick and Kutchan, 1999; Dong et al., 2003). The BMT enzymes also belong to Group II. Sev-eral identified plant OMT genes encode a universal OMT signature composed of five highly conserved regions, two of which (regions I and IV) are believed to be involved in SAM and metal binding, respectively (Ibrahim et al., 1998; Kopycki et al., 2008). Despite the fact that several plant OMT genes have already been cloned, more such se-quences are required to obtain a complete picture.
|
|
|
|
The dried root of Angelica dahurica (Umbelliferae), also known as Bai Zhi, is an important Chinese traditional herb. Many coumarins isolated from Bai Zhi exhibit antimicrobial activities (Kwon et al., 1997). Interestingly, 8-hydroxybergapten, a furanocoumarin from Bai Zhi, has potent inhibitory activities against mushroom tyrosinase (Piao et al., 2004). Thus, Bai Zhi may potentially be used to treat abnormal pigmentation disorders and applied to skin-whitening compositions in the cosmetic industry. Since 8-hydroxybergapten is a skin-whitening component, the objective of the present study is to identify BMT from A. dahurica, a key enzyme to produce bergapten as one of the two major steps towards the production of 8-hydroxy-bergapten, a skin-whitening agent. The development of E. coli as a bioreactor to produce bergapten which is superior to the use of cell culture is also discussed.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plants of Bai Zhi [Angelica dahurica (Fish.) BENTH. et HOOK] were grown in the field. Roots were collected and immediately frozen in liquid nitrogen. All the plant material was stored at -80°C until use.
|
|
|
|
|
|
|
|
Total RNA was isolated from 3 month-old roots of A. dahurica. The cDNA fragments were generated by reverse transcriptase-polymerase chain reaction (RT-PCR) amplification using a pair of degenerate primers designed from two highly conserved sequences of plant OMTs. The 5'-primer (5'-gtg/tgatgttggc/tggtggg/a/cactgga/t-3') resides in conserved region I while the 3'-primer (5'-ggg/a/tgcatcg/ t/cc/tca/gac/tg/a/cacgtga/ggg-3') in conserved region II as indicated in Figure 2. The generated cDNA fragments were cloned in pGEM-T Easy vector (Promega, Madison, WI, USA) and sequenced to confirm their identities. The tandem 5'- and 3'-rapid amplification of cDNA ends (RACE) polymerase chain reaction (PCR) was done according to the user manual of SMART™ RACE cDNA amplification kit (CLONTECH Laboratories, Inc., Mountain View, CA, USA). The complete DNA sequence was determined from both strands of cloned inserts with an ABI 3730 XL DNA analyzer (Foster City, CA, USA) by Mission Biotech Co. Ltd. (Taipei, Taiwan). Sequence alignment was achieved using the Vector NTI Suite 8 program (InforMax, Inc., Bethesda, MD, USA) and the homology search was done with the BLAST program (Altschul et al., 1997).
|
|
|
|
Figure 1. Biosynthesis pathway of bergapten and 8-hydroxybergapten.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LO et al. ― Cloning and activity of bergaptol-O-methyltransferase
|
|
|
|
|
|
|
|
|
Overexpression of AdBMT and kinetic conversion of bergaptol into bergapten in E. coli
|
(50 μ/ml) at 37°C until an OD600 between 0.6-0.8 was reached. Isopropyl p-D-thiogalactopyranoside (IPTG) was then added to give a final concentration of 1 mM and cells were then incubated at various indicated temperature conditions for additional 16 h. Samples of cells were harvested by centrifugation and either used for further protein isolation and enzyme purification or resuspended in 25 ml of LB medium containing ampicillin (50 μml) and 100 μM bergaptol and incubated for an additional 24 h at 25°C. After centrifugation, the supernatant was used to extract bergapten for measurement by ultra performance liquid chromatography (UPLC). Bergapten yield was normalized on the basis of equal portions of E. coli cells (wet weight). Only the AdBMT-overexpressing E. coli grown at the optimum induction temperature was taken for the kinetic analysis. The incubation time was extended from 24 to 48 h.
|
|
|
|
The full-size coding AdBMT cDNA as a template and a pair of gene-specific primers AdBMT-over-f
(5'-GTCGACCATATGGCAGAAATGAAAACTAG-3') containing a Sall site, and AdBMT-over-r
(5'-GCGGCCGCCTTCGAAAATTCCATAATC-3') contam-ing a NotI site were used for PCR amplifications. The 1,077 bp AdBMT fragment was therefore cloned into the SalI/NotI-cut pET32a expression vector (Novagen, Madison, WI, USA). After digestion with NdeI, the resulting 6.5 kb-fragment was eluted and self-ligated to produce an AdBMT expression plasmid designated pET32a-AdBMT that expressed AdBMT with His-tag at the C-terminus. The pET32a-AdBMTa was transformed into E. coli BL21. After inoculation, the bacterial cells were grown in the LB medium (1% Bacto tryptone, 0.5% Bacto yeast extract, and 170 mM NaCl, pH 7.0) containing ampicillin
|
|
|
|
|
|
|
|
|
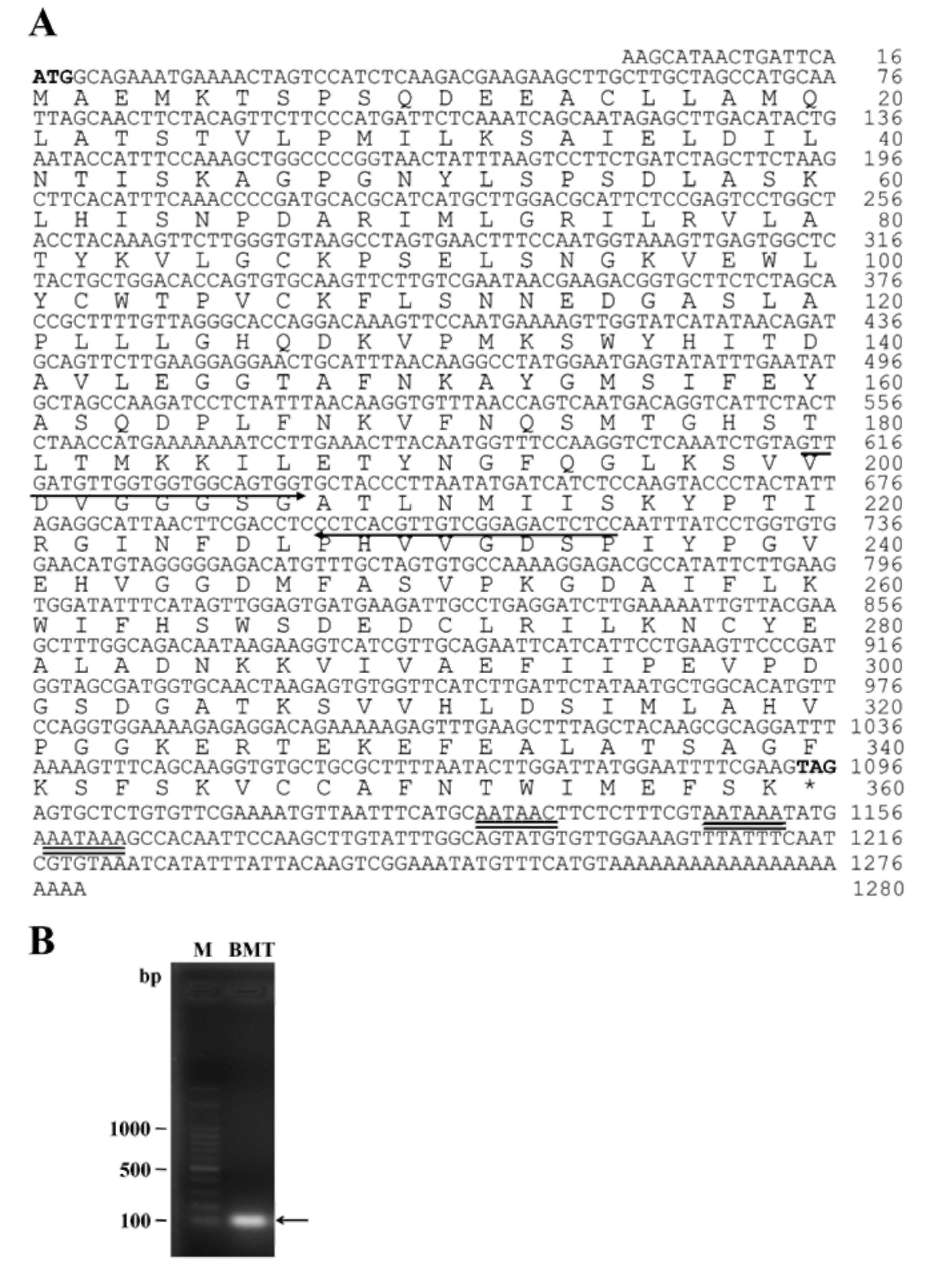
Figure 2. Nucleotide and predicted amino acid sequences of AdBMT cDNA identified from A. dahurica. (A) The numbers of the nucleotide sequence and amino acid sequence are indicated on the right. The bold letters in the nucle-otide sequence indicate start and stop codons. The two degenerate primers are indicated by arrows. The putative polyadenylation signals are double underlined. The translation end is marked with an asterisk; (B) RT-PCR was performed on total RNA isolated from A. dahurica root. The sequence of AdBMT was amplified using a pair of indicated degenerate primers.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
Ultra performance liquid chromatography
|
buffer (pH 7.5) containing 10 mM ethylenediaminetetraa-cetic acid (EDTA), sonicated and centrifuged at 16,000 xg for 5 min at 4°C. Because Ni-nitrilotriacetic acid (NTA) column is not suitable for BMT purification (Hehmann et al., 2004), total protein in the supernatant was fractionated by ammonium sulfate precipitation from which a fraction of 40-50% saturation was collected. Protein in this fraction was dissolved in 200 mM potassium phosphate buffer (pH 7.5) and desalted through PD-10 columns (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The ammonium sulfate-fractionated protein was further concentrated by centricon-30 (Millipore, Billerica, MA, USA) and its concentration was determined. Each fraction was subjected to enzyme activity assay.
|
|
|
|
After centrifugation, 5 ml aliquots of the supernatant were taken and mixed with ethyl acetate (1 ml) and centri-fuged at 16,000 xg for 2 min. The organic layer was collected and evaporated in vacuum after which the residue was dissolved in methanol (0.3 ml). The bergaptol and bergapten were then measured at indicated time intervals by UPLC.
|
|
|
|
The reaction product was analyzed using a Waters AC-QUITYTM UPLC system (Waters Crop, Milford, MA, USA) that contains a cooling autosampler, a column oven enabling temperature control of analytical column and a photodiode array detector. For each injection, 5 μl of methanol-dissolved product was loaded onto a BEH C18 (2.1 mm x 18 cm, 1.7 [im) column and eluted with a wa-ter/acetonitrile mix (70/30) by UPLC pump at a flow rate of 0.25 ml/min. The column was maintained in an oven at 40°C. Detection was at 254 nm. The reaction product was determined and quantitated in duplicate. The pure (authentic) bergaptol (ChromaDex, Inc., Irvine, CA, USA) and bergapten (Sigma-Aldrich Chemical Co., St Louis, MO, USA) were used as standard chemicals. Data were collected and processed by chromatographic software MassLynx (Waters Crop, Milford, MA, USA).
|
|
|
|
Assay of BMT (EC 2.1.1.69) activity was as previously reported (Hehmann et al., 2004) with some modifications. Briefly, BMT activity in vitro was routinely measured at 35°C in a volume of 200 μl containing 200 mM potassium phosphate buffer, pH 7.5, 1.25 mM bergaptol as a substrate (Indofine Chemical Co, Somerville, NJ, USA), and 9.6 [ig of purified protein which corresponds to 2.0 nkat BMT activity (specific activity 0.21 kat/kg). The reaction was started with the addition of SAM (5 mM) and terminated after 90 min incubation by adding 30 μl of 1N HCl. The reaction mixture was extracted with ethyl acetate (0.5 ml) and centrifuged at 16,000 xg for 2 min. The organic layer was collected and evaporated in vacuum after which the residue was dissolved in methanol (0.3 ml). The reaction product (bergapten) was then determined and quantitated using UPLC in duplicate. The extract of cells with empty vector was used as a negative control.
|
|
|
|
SDS-PAGE and immunoblotting
|
|
|
|
For protein fractionation, the cell pellets collected from 16°C were resuspended in 200 mM potassium phosphate buffer (pH 7.5) containing 10 mM ethylenediaminetetraa-cetic acid (EDTA), sonicated and centrifuged at 16,000 xg for 5 min at 4°C. Total protein in the supernatant was mixed with 2x sample buffer [2% (w/v) sodium dode-cyl sulfate (SDS) and 4% (v/v) 2-mercaptoethanol] and fractionated by 12% (w/v) SDS-polyacrylamide gel elec-trophoresis (PAGE). After electrophoresis, the gel was either stained with 0.25% Coomassie blue R-250, 10% acetic acid, and 5% ethanol for 4 h, and destained with 10% acetic acid and 20% ethanol or electroblotted onto nitrocellulose (0.45 [m, Sartorius Stedim, Biotech, Goet-tingen, Germany). The membrane was blocked at 37°C for 1 h in a solution containing 10 mM Tris-HCl, pH 7.5, 0.05% Tween-20, 150 mM NaCl and 5% nonfat dry milk. The membrane was incubated with anti-His-tag antibody with a 1:1,000 dilution for 2 h at 4°C after which the membrane was washed, incubated with alkaline phos-phatase-conjugated secondary antibody for 1 h at 4°C. The locations of antigen-antibody complex were visualized by color development catalyzed by alkaline phosphatase with 5-bromo-4-chloro-3-indolyl phosphate substrate and nitro blue tetrazolium in a carbonate buffer (100 mM NaHCO3, 1 mM MgCl2, pH 9.8). Protein concentration was deter-mined according to Bradford (1976) with bovine serum albumin as a standard.
|
|
|
|
pH and temperature optimum and metal requirement of AdBMT
|
|
|
|
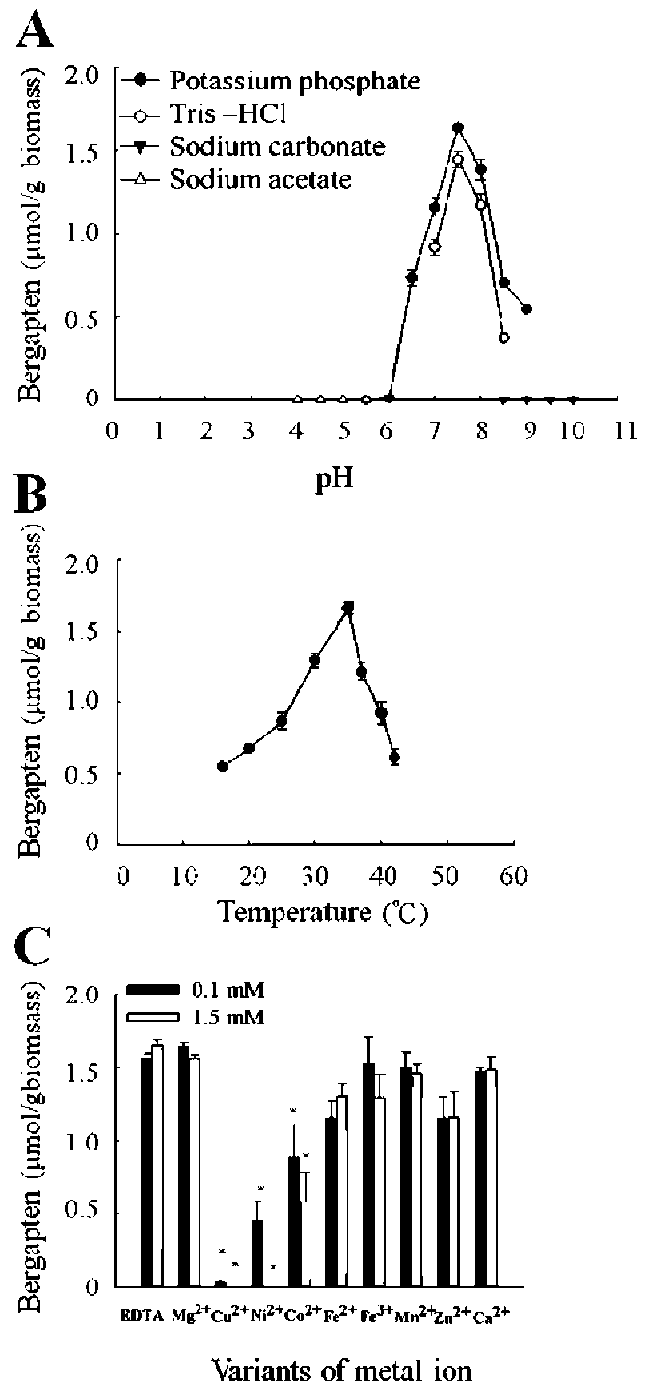
The optimal pH for enzyme activity was determined by incubations at 35°C for 90 min in different buffers (200 mM) ranging between 4.0 and 10.0 (acetate, pH 4.0-5.5; phosphate, pH 5.5-9.0; Tris-HCl, pH 7.0-8.5; carbonate, pH 8.5-10.0). The optimum temperature for enzyme activ-ity was determined between 16°C and 42°C in 200 mM potassium phosphate buffer (pH 7.5) for 90 min. The effect of various metal ions at 0.1 and 1.5 mM concentrations on enzyme activity was determined in 200 mM potassium phosphate buffer (pH 7.5) at 35°C for 90 min. The addi-tion of 5.4 mM EDTA in the buffer was used as a control. Three independent experiments in duplicate were carried out for each analysis.
|
|
|
|
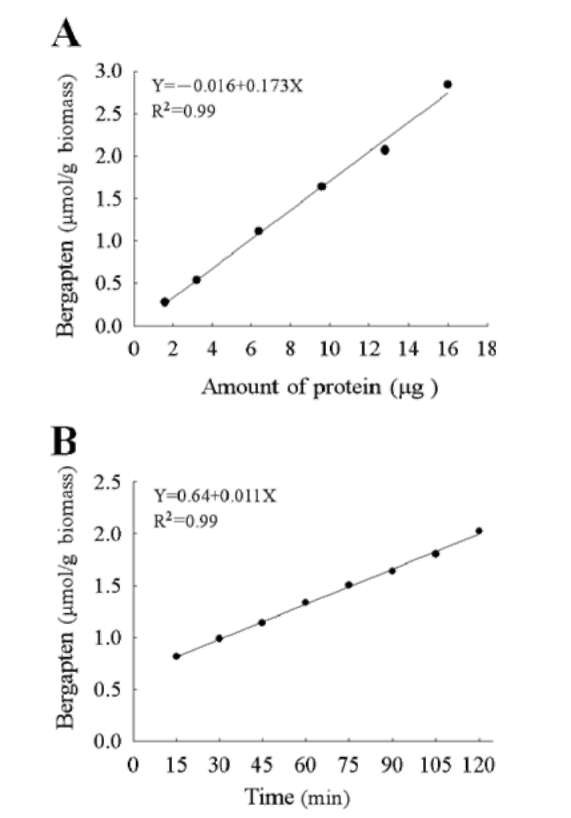
Reactions were assayed under conditions in which product formation was linear for both time (15-120 min) and the concentration of purified protein (1.8-16 μg). The apparent Km and Vmax values were determined by Lin-eweaver-Burk plots using approximately 9.6 μg desalted protein extract and the adjustment of a serial dilutions of the substrates both bergaptol (0.625~7 mM) and SAM (1.25 mM~7.5 mM) in the enzyme reaction as described above.
|
|
|
|
Enzyme purification and activity assays
|
|
|
|
For enzyme purification, the cell pellets collected from 16°C were resuspended in 200 mM potassium phosphate
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LO et al. ― Cloning and activity of bergaptol-O-methyltransferase
|
|
|
|
|
|
|
|
|
|
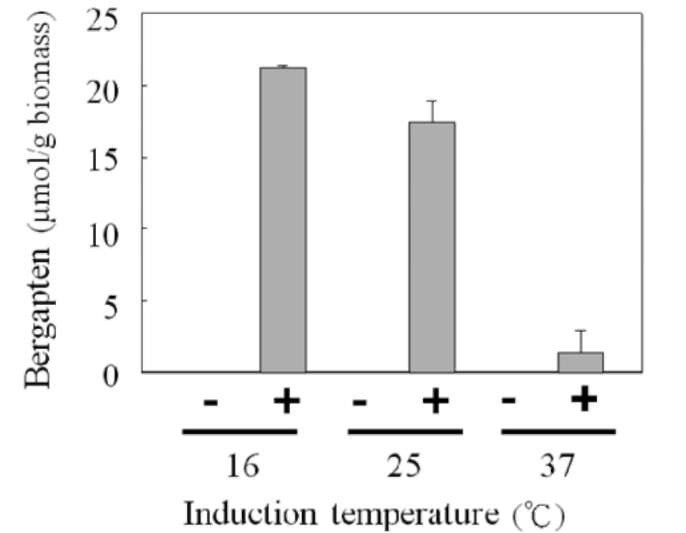
as the induction temperatures decreased (Figure 4). The maximal amount of bergapten was produced in incubations at 16°C, indicating the most suitable setting for highest AdBMT activity among the test temperatures. Therefore, 16°C was chosen for further investigation. Controls with an empty vector were conducted in a parallel manner at each incubation temperature; no bergapten was detected in any of the control experiments (Figure 4).
|
|
|
|
Cloning and characterization of BMT cDNA from Bai Zhi
|
|
|
|
To obtain the BMT cDNA from Bai Zhi, total RNA isolated from Bai Zhi root was used as a template. A pair of degenerate oligonucleotide primers were designed from highly conserved regions of other plant BMT sequences as indicated in Figure 2A. Using RT-PCR amplifications, a single band of 107 bp DNA fragment corresponding to AdBMT sequence was obtained (Figure 2B). Because the cDNA size was partial, the method of 5'- and 3'-RACE-PCR was used to obtain the full length of 1,259 bp AdBMT cDNA (accession no. JN585954) excluding the poly (A) tail.
|
|
|
|
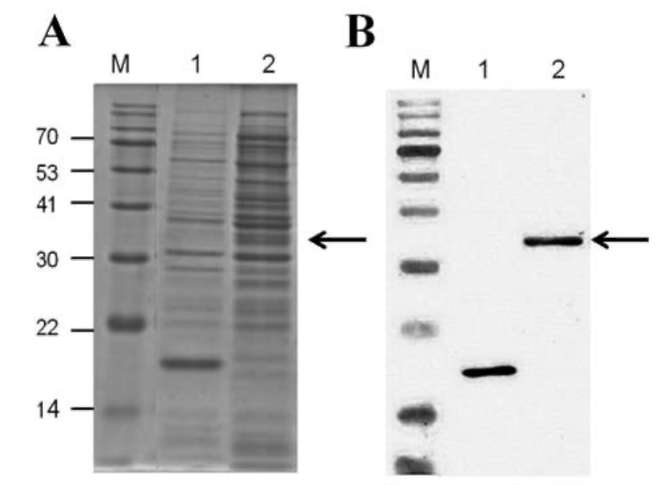
The protein from each preparation was further separated by SDS-PAGE (Figure 5A). A 39-kDa protein was observed in cells harboring pET32a-BMT (lanes A2), whereas it was not detected in cells harboring empty pE-T32a (lane A1), suggesting that the recombinant AdBMT (with His-tag) was successfully expressed in E. coli. The AdBMT protein was immunologically detected using anti-His-tag antibody, as indicated by an arrow (Figure 5B, lane 2). The sizes of the detected proteins were all in good agreement with the expected size of 39 kDa predicted from the AdBMT sequence. The 17.5-kDa band produced by a pET32a vector was used as a control (Figure 5, lane 1).
|
|
|
|
The AdBMT cDNA contained an open reading frame of 1080 bp encoding a polypeptide of 359 amino acids (Figure 2A) with a calculated molecular mass of 39 kDa and a calculated pI of 5.9. Assessment of its hydropathy profile (Kyte and Doolittle, 1982) showed that the AdBMT polypeptide did not have a strong hydrophobic region near the N-terminus, possibly indicating the absence of a signal peptide (data not shown). In the 3'-untranslated region, a variant AATAAC and two putative AATAAA consensus motifs of polyadenylation were located at 130, 113, and 102 bp upstream from the site of polyadenylation.
|
|
|
|
Characterization of AdBMT
|
|
|
|
Because Hehmann et al. (2004) reported that the BMT activity of A. majus in the bacterial extracts was very labile and could not enable the extensive purification, the AdBMT extract isolated from the E. coli cells was thus, subjected to ammonium sulfate fractionation (40%-50% saturation) and subsequently desalted and concentrated for biochemical characterization.
|
|
|
|
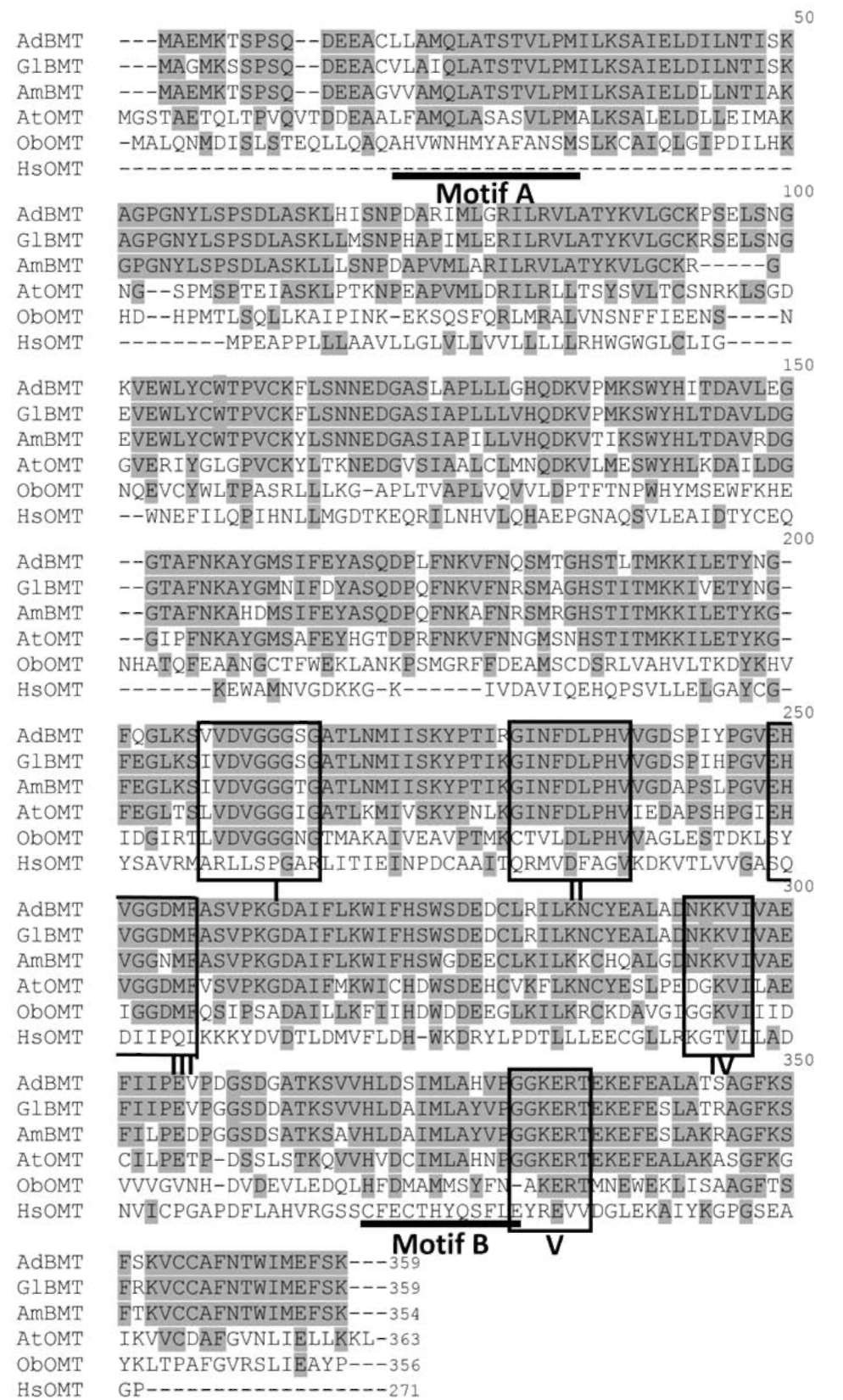
The predicted amino acid sequence of AdBMT was used to search protein databases. Sequence alignment revealed that the AdBMT polypeptide, in which all five conserved regions I-V (Ibrahim et al., 1998) were found, shared 28%-91% identity with plant OMTs. and identified the least with human OMT (13%) (Figure 3). AdBMT exhib-its high sequence similarity with GlBMT (91% identity) and AmBMT (84% identity). Regions I and IV had been shown by X-ray diffraction to be involved in SAM and metal binding, respectively (Vidgren et al., 1994). In ad-dition to the common signature of these highly conserved regions (regions I-V) characterized for SAM-dependent OMTs, motifs A and B were considered to govern the sub-strate specificity (Joshi and Chiang, 1998; Schroder et al., 2002).
|
|
|
|
As shown in Figure 6, bergapten formation was linear for both indicated time (15-120 min) and concentrations of purified protein (1.8-16 ig). Therefore, 9.6 [ig of purified protein (specific activity 0.21 kat/kg) and 90 min of reaction time at 35°C were taken to measure the optimal pH of AdBMT activity. It should be noted that the bergapten production reached a maximum level (36 [g) after 2-h incubation (Figure 6B). Significant activity of the recombinant AdBMT was observed from pH 6.5-9.0 with an optimum pH of around 7.5 either in potassium phosphate or in Tris-HCl buffer (Figure 7A). At pH 7.5, the AdBMT activity in the potassium phosphate buffer was active over a broad temperatures ranging from 16 to 42°C with an optimum temperature of around 35°C (Figure 7B). It should be not-ed that without the addition of SAM (5 mM), no bergapten was observed in the reaction mixture. The kinetic analysis revealed that the Km value of AdBMT to bergaptol is 0.56 mM whereas the Km value of AdBMT to SAM is 10.68 mM.
|
|
|
|
Overexpression of recombinant AdBMT in E. coli
|
|
|
|
The open reading frame sequence of AdBMT was subcloned into an expression vector, pET32a, and over-expressed in E. coli. To determine the conditions for high functional AdBMT expression, cultures of the transfor-mants were induced at various temperatures for 16 h in the presence of 1 mM IPTG. The E. coli cells were harvested by centrifugation and resuspended in LB medium for 24 h incubation with 100 [M bergaptol, and the formation of the product (bergapten) was quantified by UPLC as a biotransformation assay for cellular AdBMT activity. The overexpressed AdBMT in the cell may convert bergaptol into bergapten, which could be identified and quantitated by UPLC. Bergapten production significantly increased
|
|
|
|
Plant OMTs can be categorized into two major classes, Class I and Class II. Class I is Mg2+-dependent, while Class II does not require Mg2+ for catalytic activity (Joshi and Chiang, 1998; Kopycki et al., 2008). The effect at 0.1 or 1.5 mM concentrations of Mg2+ on enzyme activity was examined in the potassium phosphate buffer (pH 7.5) at 35°C for 90 min. No matter which concentration of Mg2+ was applied to the incubation, the production of bergapten
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 3. Amino acid alignment of the AdBMT protein sequence with other BMTs and OMT-related proteins. A dash in the sequence indicates a gap introduced to maintain good alignment. The five highly conserved regions (regions I-V) are indicated by a box. The motifs A and B are indicated by an underline. The two BMTs are GlBMT (AB363638), a bergaptol O-methyltransferase of Glehnia littoralis, and AmBMT (AY443006), a bergaptol O-methyltransferase of Ammi majus. The OMT-related proteins include AtOMT (NM_124796), a quercetin 3-O-methyltransferase of Arabidopsis thaliana; ObOMT (AB530137), a chavicol O-methyltransferase of Ocimum basilicum; and HsOMT (Z26491), a catechol O-methyltransferase of Homo sapiens.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LO et al. ― Cloning and activity of bergaptol-O-methyltransferase
|
|
|
|
|
|
|
|
|
was similar to that in the medium containing EDTA, a metal chelator, as a control, suggesting that AdBMT does not require Mg2+. This result is consistent with that of AmBMT, whose catalytic activity is reported to be Mg2+-independent (Hehmann et al., 2004).
|
|
|
|
|
The effect of several other metal ions (Cu2+, Ni2+, Co2+, Fe2+, Fe3+,' Mn2+, Zn2+, and Ca2+) on enzyme activity was also examined. Significant inhibition of AdBMT activity was observed in the presence of Cu2+ (99% and 100%), Ni2+ (71% and 100%), and Co2+ (50% and 64%) (Figure 7C). The addition of Cu2+ and Ni2+ up to 1.5 mM completely inhibited AdBMT activity. Fe2+ and Zn2+ only slightly affected AdBMT activity at 0.1 mM concentration. However, the addition of Mn2+, Fe3+, and Ca2+ barely af-fected AdBMT catalytic activity (Figure 7C).
|
|
|
|
|
|
|
|
Efficient production of bergapten in the culture overexpressing AdBMT
|
Figure 5. Overexpression of AdBMT in E. coli. The cells harboring empty pET32a (1) or pET32a-BMT (2) plasmids were expressed in the presence of 1 mM IPTG for 16 h at 16°C. The total protein was extracted from each preparation. An equal volume of protein was separated by SDS-PAGE and either stained with Coomassie blue (A) or electroblotted onto nitrocellulose and immunologically detected using anti-His-tag antibody (B). M indicates marker proteins (70, 53, 41, 30, 22, and 14 kDa). The arrows indicate the overexpressed AdBMT.
|
|
|
|
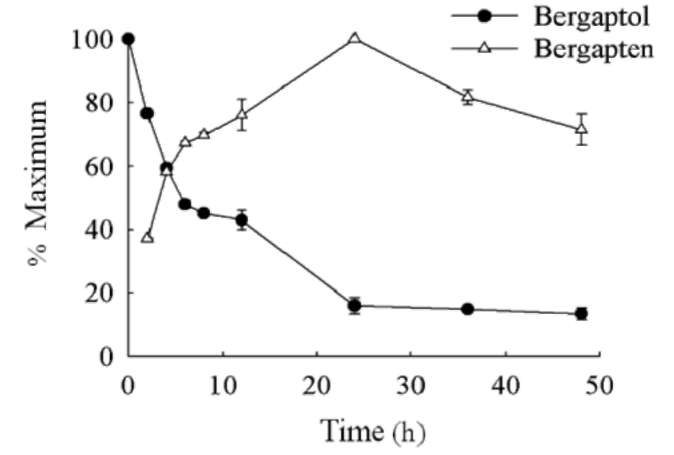
The conversion kinetics of bergaptol into bergapten in the culture overexpressing AdBMT was further examined. After the E. coli cells were grown at 16°C for 16 h, the cells were harvested by centrifugation and resuspended in LB medium, When bergaptol was added to give a final concentration of 100 fM, the cells were incubated at 25°C for 48 h (Figure 8). For bergaptol conversion, 48 h was used instead. After a short reaction period of 2 h, the production of bergapten reached 180 [g, which corresponds to 60% of the maximal level observed after 24 h if the maximum level of bergapten production at 24 h was
|
|
|
|
|
|
|
|
|
|
|
|
Figure 4. Estimation of the functional AdBMT overexpressed in E. coli. Fifty milliliters of cell culture overexpressing AdBMT and an empty vector were induced in the presence of 1 mM IPTG at the indicated temperatures for 16 h. The cells from each preparation were harvested by centrifugation, resuspended in 25 ml of LB and incubated at 25°C for 24 h with the addition of bergaptol to achieve a final concentration of 100 !M. The product (bergapten) in the reaction medium was estimated by UPLC. Bergapten is normalized on the basis of an equal amount of E. coli cells. Three independent experiments in duplicate were carried out for each analysis.
|
|
|
|
Figure 6. Bergapten production was linear for both indicated concentrations of purified protein and time period of reaction. Bergapten was estimated in a volume of 200 [il at 35°C (A) for 90 min with the addition of various amounts of purified protein or (B) for various indicated time intervals with 9.6 [ig of purified protein (specific activity 0.21 kat/kg) isolated from AdBMT-overexpressed E. coli cells. Bergapten is normalized on the basis of an equal amount of E. coli cells.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 8. Efficient production of bergapten in the culture overexpressing AdBMT. Fifty milliliters of cell culture overex-pressing AdBMT were induced in the presence of 1 mM IPTG at 16。C for 16 h. The cells were harvested by centrifugation, resuspended in 25 ml of LB medium and incubated at 25。C for 48 h with the addition of bergaptol to achieve a final concentration of 100 [M. Various time-intervals were taken to estimate the concentration of bergapten and bergaptol in the medium by UPLC. Bergapten is normalized on the basis of an equal amount of E. coli cells. The maximum production of bergapten at 24 h is indicated as 100%. Three independent experiments in duplicate were carried out for each analysis.
|
|
|
|
counted as 100% (Figure 8). It is worthy of noting that under the same reaction time (i.e. 2 h) the production of bergapten was 5-fold higher than that produced by the ammonium sulfate-purified AdBMT fraction (36 ig) derived from the same amount of cells. When the incubation time extended to 24 h, the production of bergapten reached a maximum yield of 460.1 [g, which was approximately 13fold higher than that produced by the ammonium sulfate-precipitated AdBMT fraction that is also derived from the same amount of cells (Figure 8). Afterwards, the concentration of the bergapten gradually decreased to 71% of conversion after 48 h of incubation.
|
|
|
|
|
|
|
|
Bai Zhi, a Chinese herb, has long been used as a face cream for skin-whitening purposes. The mechanism of skin-whitening compositions was not scientifically proven until Piao et al. (2004) reported that ethyl acetate extracts of A. dahurica that had potential inhibitory activity against mushroom tyrosinase. Tyrosinase is a rate-limiting enzyme that converts tyrosine to 3,4-dihydroxyphenylalanine and subsequently oxidizes it to form dopaquinone, which leads to the ultimate formation of melanin. Considering that ty-rosinase inhibitors prevent the formation of melanin, they may result in reductions in skin darkness. The active skin-whitening material isolated from Bai Zhi root was identified as 8-hydroxybergapten (Piao et al., 2004), which is a compound converted from bergapten by one-step hydroxy-lation. Bergapten, however, is the product from bergaptol
|
|
|
|
Figure 7. Effects of pH, temperature and metal ions on the recombinant AdBMT activity. Approximately 9.6 [g of ammonium sulfate-fractionated protein were added into (A) 200 mM of various indicated buffers which cover a wide pH range between pH 4.0 and 10.0, (B) into 200 mM potassium phosphate buffer (pH 7.5) and incubated at indicated temperatures from 16 to 42。C, or (C) into 200 mM potassium phosphate buffer (pH 7.5), all of which contain various metal ions at 0.1 and 1.5 mM concentrations, and incubated at 35。C, respectively. EDTA, a metal chelator, was added to the buffer as a control. Bergapten is normalized on the basis of an equal amount of E. coli cells. Three independent experiments in duplicate were carried out for each analysis. The asterisks indicate the significance of the difference from the control value determined by the Student's t-test (*P < 0.01). The error bars represent SD.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LO et al. ― Cloning and activity of bergaptol-O-methyltransferase
|
|
|
|
|
|
|
|
|
catalyzed by BMT, a key enzyme of the furanocoumarin biosynthesis pathway.
|
independent (Figure 7C). However, the addition of Cu , Ni2+, and Co2+ at concentrations even as low as 0.1 mM severely inhibited enzyme activity. Similar results were described for heterologous OMTs (Morishige et al., 2000; Hehmann et al., 2004). In addition, the loss of enzyme activity may also due to the following purification processes such as elution and each concentrated step. Given that Ni2+ ions inhibit BMT activity, the Ni-NTA column commonly used to purify protein is not suitable for BMT purification. Thus, ammonium sulfate precipitation is adopted for the purification of AdBMT from protein extracts of AdBMT-overexpressing cells.
|
|
|
|
A earlier report indicated that BMT is labile and thus, the method of ammonium sulfate fractionation is adopted for enzyme purification (Hehmann et al., 2004). Accordingly, AdBMT was routinely isolated from E. coli cell culture and purified by ammonium sulfate fractionation. A simple and efficient method is developed to produce bergapten in E. coli (Figure 8). The production of bergapten in vivo has several major advantages. First, given the same amount of E. coli cells, a large amount of bergapten can be produced in the fluid. After a short reaction period of 2-h (Figure 8), the production of bergapten in vivo is 5-fold higher than that produced by the ammonium sulfate-purified AdBMT fraction derived from the same amount of cells. More importantly, the incubation of the medium can be further extended to 24 h to reach maximal production of bergapten, but not for the reaction that is conducted in vitro because the exogenous SAM and added AdBMT may be labile. The production of bergapten in vivo is, thus, 13fold higher than that produced by the ammonium sulfate-purified AdBMT fraction (Figure 8). These results suggest that the ammonium sulfate-purified AdMBT fraction significantly decreases its enzyme activity probably due to the processes of protein extraction and purification. Second, SAM is no longer necessary to be added into the reaction in vitro because E. coli can self-supply the cofactor for methylation and thus, greatly reduce production costs. Third, the purification of BMT from the cells is unnecessary. E. coli can utilize the supplemented bergaptol and convert it into bergapten, which is finally released to the medium although the secretory mechanism of bergapten into the medium is unclear. Given the fact that bergapten can be detected in the medium, the isolation and purification of bergapten is thus greatly simplified. It is consistent with a previous report published by Ishikawa et al. (2009), who indicated that bergapten can be produced in the fluid of G. littoralis cell suspension cultures. However, the production of bergapten in E. coli cell culture is more convenient than that produced in the fluid of G. littoralis cell suspension because of the preparation of E. coli is easy and widely used.
|
|
|
|
In conclusion, the BMT cDNA was identified from Bai Zhi and overexpressed in E. coli. The optimum conditions of AdBMT were determined in potassium phosphate buffer, and it was found that the enzyme does not require Mg2+ for catalytic activity. A simple and efficient production of bergapten in the culture fluid was developed. With the supply of bergaptol in the medium, E. coli cells can be used as a potential bioreactor to produce bergapten.
|
|
|
|
Acknowledgments. This work was supported by National
Science Council Grant NSC98-2311-B-005-002-MY3 in
Taiwan to C.-S. Wang.
|
|
|
|
|
|
|
|
Alexander, N.J., S.P. McCormick, and J.A. Blackburn. 2008. Effects of xanthotoxin treatment on trichothecene production in Fusarium sporotrichioides. Can. J. Microbiol. 54: 10231031.
Altschul, S.F., T.L. Madden, A.A. Schaffer, J. Zhang, Z. Zhang,
W. Miller, and D.J. Lipman. 1997. Gapped BLAST and
PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389-3402.
Bradford, M. 1976. A rapid and sensitive method for the quanti-tation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254.
Dong, C., D. Anzellotti, R.K. Ibrahim, N.P. Huner, and F.
Sarhan. 2003. Daphnetin methylation by a novel O-meth-yltransferase is associated with cold acclimation and pho-tosystem II excitation pressure in rye. J. Biol. Chem. 278:
6854-6861.
Frick, S. and T.M. Kutchan. 1999. Molecular cloning and functional expression of O-methyltransferases common to iso-quinoline alkaloid and phenylpropanoid biosynthesis. Plant J. 17: 329-339.
Hehmann, M., R. Lukacin, H. Ekiert, and U. Matern. 2004. Furanocoumarin biosynthesis in Ammi majus L. Cloning of bergaptol O-methyltransferase. Eur. J. Biochem. 271: 932940.
Ibrahim, R.K., A. Bruneau, and B. Bantignies. 1998. Plant O-methyltransferases: molecular analysis, common signature and classification. Plant Mol. Biol. 36: 1-10.
Ishikawa, A., T. Kuma, H. Sasaki, N. Sasaki, Y. Ozeki, N. Ko-
|
|
|
|
The amino acid sequence of AdBMT shares high sequence similarity with the BMT sequences from G. lit-toralis and A. majus. In general, all plant OMTs contain five highly conserved regions (regions I-V) characterized for SAM-dependent OMTs (Vidgren et al., 1994; Ibrahim et al., 1998). The two motifs A and B are considered to control the substrate specificity (Joshi and Chiang, 1998; Schroder et al., 2002). The existence of highly conserved motifs A and B in these three BMT may reflect that they utilize the same substrate (i.e., bergaptol). This is supported by the fact that AmBMT from A. majus also uses ber-gaptol as a substrate. However, these common structural features are hardly found in animal OMTs (i.e., human).
|
|
|
|
AdBMT is classified as a member of Class II OMTs (Kopycki et al., 2008) and its enzyme activity is Mg2+-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
bayashi, and Y. Kitamura. 2009. Constitutive expression of bergaptol O-methyltransferase in Glehnia littoralis cell cultures. Plant Cell Rep. 28: 257-265.
Joshi, C.P. and V.L. Chiang. 1998. Conserved sequence motifs in plant S-adenosyl-L-methionine-dependent methyltrans-
ferases. Plant Mol. Biol. 37: 663-674.
Kopycki, J.G., D. Rauh, A.A. Chumanevich, P. Neumann, T. Vogt, and M.T. Stubbs. 2008. Biochemical and structural analysis of substrate promiscuity in plant Mg2+-dependent
O-methyltransferases. J. Mol. Biol. 378: 154-164.
Kuete, V., R. Metuno, B. Ngameni, A.M. Tsafack, F. Ngandeu, G.W. Fotso, M. Bezabih, F.X. Etoa, B.T. Ngadjui, B.M. Abegaz, and V.P. Beng. 2007. Antimicrobial activity of the methanolic extracts and compounds from Treculia obovoid-ea (Moraceae). J. Ethnopharmacol. 112: 531-536.
Kwon, Y.S., A. Kobayashi, S.I. Kajiyama, K. Kawazu, H. Kan-zaki, and C.M. Kim. 1997. Antimicrobial constituents of Angelica dahurica roots. Phytochemistry 44: 887-889.
Kyte, J. and R.F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:
|
105-132.
Morishige, T., T. Tsujita, Y. Yamada, and F. Sato. 2000. Molecular characterization of the S-adenosyl-Lmethionine: 3'-hydroxy-N-methylcoclaurine 4'-O-methyltransferase involved in isoquinoline alkaloid biosynthesis in Coptis japonica. J. Biol. Chem. 275: 23398-23405.
Piao, X.L., S.H. Baek, M.K. Park, and J.H. Park. 2004. Tyro-
sinase-nhibitory furanocoumarin from Angelica dahurica.
Biol. Pharm. Bull. 27: 1144-1146.
Schroder, G., E. Wehinger, and J. Schroeder. 2002. Predicting the substrates of cloned plant O-methyltransferases. Phy-tochemistry 59: 1-8.
Tietjen, K.G., D. Hunkler, and U. Matern. 1983. Differential
response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur. J. Biochem. 131:
401-407.
Vidgren, J., L.A. Svensson, and A. Liijas. 1994. Crystal structure of catechol O-methyltransferase. Nature 368: 354-358.
|
|
|
|
|
|
|
|
白芷佛手柑內醇甲基轉移酶之分子選殖和功能分析以及 利用大腸桿菌有效率的量產佛手柑內酯
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1國立中興大學生物科技學研究所
2行政院農業委員會農業試驗所農業化學組
|
|
|
|
|
|
|
|
白芷中草藥古早以來具有面霜潤膚之美白功效。其中已知的一個美白成分,氫氧基化佛手柑內酯
(8-hydroxybergapte)是佛手柑內酯(bergapten)氫氧基化的產物,而佛手柑內酯是由佛手柑內醇甲基轉移
酶(bergaptol 5-O-methyltransferase, BMT)轉化佛手柑內醇(bergaptol)而成。從來自於其他植物BMT高
保留區的序列中設計一對返化引子(degenerate primers),再從白芷根中選殖出單一的AdBMT DNA片
段。利用5'-與3'-RACE-PCR對白芷DNA片段進行聚合酶連鎖增幅反應以取得全長的cDNA序列。
此AdBMT可編譯區爲1,080個核苷酸'可轉譯出359個胺基酸之蛋白質'預測分子量爲39 kDa 。經序
列比對分析顯示AdBMT和其它植物O-methyltransferases具有相當程度的相同度(identity)'序列中含有
的I-V區域全部保留。將AdBMT轉形至大腸桿菌可表達出含His-tag的融合蛋白質'進一歩硫酸胺鹽
沈澱以局部純化蛋白質。AdBMT於pH 7.5之磷酸鉀緩衝液和35。C的反應溫度下的活性最佳,且其活
性不須要二價離子的存在。AdBMT的活性受到Cu2+ 、Ni2+及Co2+等離子的嚴重抑制,即使濃度低至0.1
mM 。在培養液中含有AdBMT基因之大腸桿菌可簡便有效率的轉化佛手柑內醇(bergaptol)成大量的佛
手柑內酯,其產量比經過硫酸胺鹽純化之酵素所能催化的量還多出13倍。大腸桿菌可成爲有潛力的生
物反應器,以生產佛手柑內酯。
|
|
|
|
|
|
|
|
關鍵詞:白芷;佛手柑內酯;佛手柑內醇甲基轉移酶;cDNA選殖;酵素活性。
|
|
|
|
|
|
|
|
|
|
|