|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies (2012) 53: 223-227
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Photosynthetic properties and photosystem stoichiometry of in vitro-grown juvenile, adult, and rejuvenated Sequoia sempervirens (D. Don) Endl.
|
|
|
|
|
|
|
|
Hsiu-An CHU1, Ing-Feng CHANG2, Chin-Hui SHEN2, Yu-Ting CHEN3, Hsing-Ting WANG1,
Li-Chun HUANG1*, and Kai-Wun YEH2 *
|
|
|
|
|
|
|
|
1Institute of Plant and Microbial Biology, Academia Sinica, Taipei, 11529, Taiwan
2Institute of Plant Biology, National Taiwan University, Taipei 106, Taiwan
3Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung 402, Taiwan
|
|
|
|
|
|
|
|
(Received November 29, 2011; Accepted March 7, 2012)
|
|
|
|
|
|
|
|
ABSTRACT. Photosynthetic properties and photosystem stoichiometry of in vitro-grown juvenile, adult, and rejuvenated Sequoia sempervirens shoots were characterized. 77K fluorescence analysis indicated that photosystem II/photosystem I ratios were highest for chloroplasts in adult shoots. Photosynthetic oxygen evolution rates (on the same chlorophyll bases) were also slightly higher for chloroplasts in adult shoots. Our results suggested a significant alteration of photosystem stoichiometry in chloroplasts of Sequoia sempervirens shoots during phase change. In addition, chlorophyll a fluorescence analysis showed that juvenile, adult, and rejuvenated shoots showed virtually identical maximal quantum efficiencies of photosystem II (Fv/FM). Nonpho-tochemical quenching (NPQ) during actinic light illumination, however, was significantly enhanced for AS76 adult shoots. The differences in photosynthetic properties and photosystem stoichiometry among juvenile, adult, and rejuvenated shoots may reflect adjustments in the photosynthetic apparatus to acclimate to distinct physiological states of Sequoia sempervirens during phase changes.
|
|
|
|
|
|
|
|
Keywords: Nonphotochemical quenching; Phase change; Photosystem stoichiometry; Photosynthesis; Sequoia sempervirens.
|
|
|
|
|
|
|
|
|
Growth rate, shoot elongation, and rates for photosynthesis and respiration (determined by fresh weight) were all higher for the juvenile and rejuvenated S. sempervirens shoots (Huang et al., 2003b). The photosynthetic rates were associated with more chlorophyll, especially chlorophyll a, in the juvenile and rejuvenated shoots. However, almost identical maximal quantum efficiency of PSII photochemistry (FV/FM) in juvenile, adult, and rejuvenated tissues indicated that the photosystems were operating with equal efficiency (Huang et al., 2003b).
|
|
|
|
Plants undergo sequential and distinct developmental stages, or phase changes, during their life cycle. The process normally begins with a strictly vegetative juvenile phase and culminates in the sexually reproductive mature adult phase. In trees, this phase change process can take several years. Sequoia sempervirens (D. Don) Endl, a coastal redwood, can be rejuvenated through repeatedly grafting its adult-phase shoot tips onto juvenile rootstocks in vitro (Huang et al., 1992). The rejuvenated shoots completely restored rooting competence and renewed vigor of roots and shoots (Huang et al., 1992). Apparent changes in esterase and peroxidase isozymes (Huang et al., 1996), different tyrosine phosphorylation patterns, and higher total protein phosphorylation were also observed in juvenile and rejuvenated shoots (Kuo et al., 1995 and Huang et al., 2003 a). Juvenile and adult phase shoots also showed restriction fragment length polymorphism of mitochondrial DNA (Huang et al., 1995).
|
|
|
|
In this work, we characterized photosynthetic activities and photosystem stoichiometry of intact chloroplasts from juvenile, adult, and rejuvenated S. sempervirens shoots. We also monitored PSII photochemistry and thermal energy dissipation on these shoots using chlorophyll a fluorescence analysis. The differences in photosynthetic properties and photosystem stoichiometry of juvenile, adult, and rejuvenated S. sempervirens shoots and their physiological significances are addressed.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sequoia shoots at different developmental stages or
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
phases were cultured as previously described (Huang et al., 1992). The adult shoot stocks (AS76 and AS94) were established by culturing shoot tips excised in 1976 and 1994, respectively. Cultures of juvenile shoot cuttings maintained as shoot stocks were established using fresh in vitro-germinated seeding. Rejuvenated shoots (RS76 and RS94) were obtained by repeatedly grafting the shoot tips five times from the two adult Sequoia shoots (AS76 and AS94) by grafting the adult shoot tips onto juvenile rootstocks. After each grafting, shoots were severed and maintained in stocks by sub-culturing 2-3 cm terminal sections every 2 months in MS-based medium (Murashige and Skoog, 1992). Sequoia shoots were grown under 6065 [imol quanta/(m2-s) light intensity.
|
[(Fm-Fo)/Fm] ratio is a measure of the maximal quantum efficiency of PSII photochemistry (Baker, 2008). In illuminated samples, the maximum fluorescence yield, Fm' is observed. Non-photochemical quenching, NPQ = (Fm-Fm')/Fm', estimates changes in the apparent rate constant for excitation decay by heat loss induced by light relative to this rate constant in the dark (Baker, 2008). A moderate actinic light intensity [about 80 [imol quanta/(m2-s)] was applied during NPQ measurement in order to assure light limitation at steady state illumination.
|
|
|
|
Measurement of fluorescence at 77 K
|
|
|
|
Fluorescence emission spectra were recorded with a fluorescence spectrometer (Jasco model FP-6500). All the measurements were carried out at 77 K using chloroplasts at a chlorophyll concentration of 50 [g/ml. The excitation wavelength used to excite chlorophyll was 435 nm (excitation bandwidth 5 nm, emission bandwidth 1 nm).
|
|
|
|
Isolation of intact chloroplasts
|
|
|
|
40 g shoot tissues (stem with leaves) were homogenized in 500 ml chloroplast (CP) buffer (50 mM Hepes-KOH, 1 mM MgCl2, 1 mM MnCl?, 2 mM EDTA, 0.5 M sucrose, pH 8.0) with a pre-cooled blender. Homogenate was filtered through two layers of miracloth and centri-fuged at 200 g for 10 min. Supernatant was collected and centrifuged at 9870 g, and stop when reached at 9870 g. Supernatant was decanted and the pellet was re-suspended in 5 ml CP buffer. The suspension was loaded on a Percoll gradient (20-40-60%) and centrifuged in a swing bucket rotor at 4776 g for 15 min. Dark green intact chloroplast bands were collected at 20/40 and 40/60% interface. The intact chloroplast fractions were diluted with 4-6 volumes of CP buffer and centrifuged at 4471 g for 5 min. The pellet was re-suspended with a small volume of CP buffer.
|
|
|
|
|
|
|
|
Isolation of intact chloroplasts
|
|
|
|
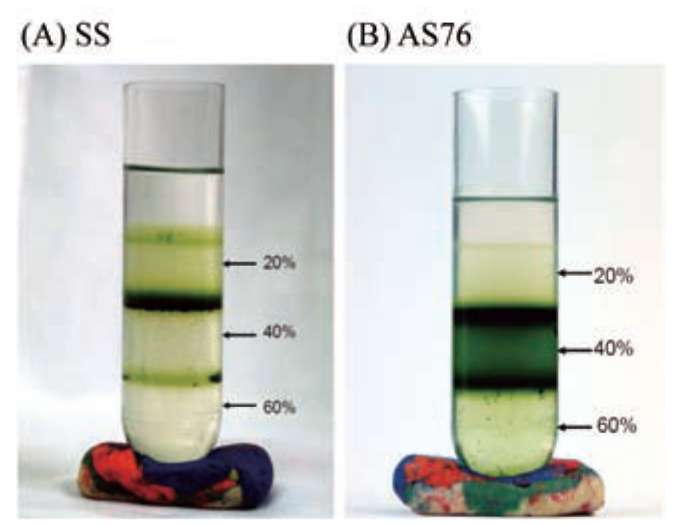
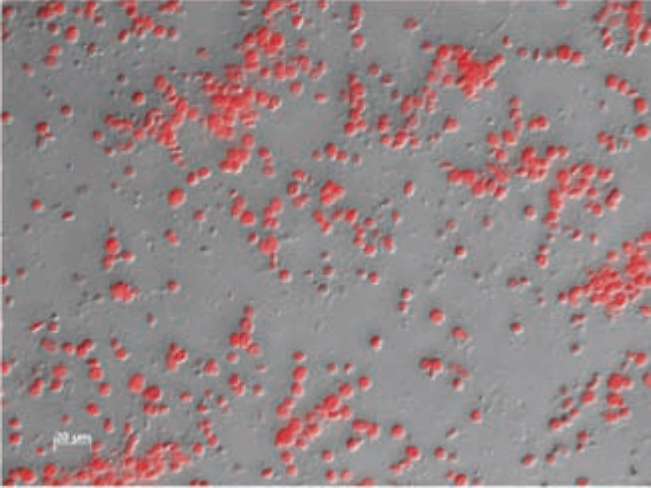
Figure 1 shows the separation of intact chloroplasts (dark green bands) from S. sempervirens shoots on a Per-coll gradient (20-40-60%). Intact chloroplasts of juvenile, adult, and rejuvenated S. sempervirens shoots were located at 20/40 and 40/60% interface. The intactness of isolated chloroplasts was routinely checked by the microscope. Figure 2 shows a microscopy photograph of isolated intact chloroplasts from S. sempervirens shoots. Red auto-fluorescence of chlorophyll signified the images of chloro-plasts. Isolated chloroplasts were mostly intact and their sizes were in the range of 5-10 fim.
|
|
|
|
Measurement of photosynthetic oxygen evolu-tion
|
|
|
|
|
|
|
|
Steady state rates of oxygen evolution were measured with a Clark-type oxygen electrode (YSI model 5331 oxygen probe) fitted with a water-jacketed cell at 25°C (Hung et al., 2010). The oxygen evolution of isolated chloro-plasts (32 [ig of Chl ml-1) was measured in CP Buffer and in the presence of 2 mM potassium ferricyanide and 2 mM 2, 6-dichloro-p-benzoquinone (DCBQ) as electron acceptors. Saturating illumination [about 2000 [imol quanta/ (m2-s)] was provided from both sides of the water-jacketed cells by two fiber-optics (Dolan-Jenner model MI 150) illuminators.
|
Photosynthetic properties of intact chloroplasts
|
|
|
|
Table 1 shows photosynthetic O2 evolution of intact chloroplasts from juvenile, adult, and rejuvenated S. sem-
|
|
|
|
|
|
|
|
Measurement of chlorophyll a fluorescence
|
|
|
|
Chlorophyll a fluorescence measurements were performed with a dual pulse-amplitude-modulation fluorom-eter (Walz) at room temperature. Just prior to fluorescence measurements, the shoots were placed in darkness for 5 min. The dark fluorescence yield (F。) in the dark-adapted state was measured by exciting with the pulse-modulated measuring light with sufficient low intensity not to induce significant variable fluorescence. Each shoot tip was then given one flash (0.6 s) of saturated pulse light to obtain the maximal (Fm) fluorescence value. The dark-adapted Fv/Fm
|
|
|
|
Figure 1. Separation of intact chloroplast (dark green bands) of Sequoia sempervirens shoots by Percoll gradients. (A) from juvenile shoots (SS); (B) from adult shoots (AS76).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHU et al. ― Phase change and photosynthesis in Sequoia sempervirens
|
|
|
|
|
|
|
|
|
pervirens shoots. Photosynthetic O2 evolution rates (on the same amount of chlorophyll basis) were significantly higher for chloroplasts from adult (AS76 and AS94) shoots than from juvenile (SS) and rejuvenated (RS76 and RS94) ones. Photosynthetic O2 evolution rates for chloroplasts from adult (AS76 and AS94) shoots ranged from 143 to 155 fimoles of O2 per mg of chlorophyll per hour. The O2 evolution rates for chloroplasts from juvenile (SS) and rejuvenated (RS76 and RS94) ones were 138, 128 and 120 fimoles of O2 per mg of chlorophyll per hour, respectively.
|
|
|
|
|
Fluorescence emission spectroscopy at 77K
|
|
|
|
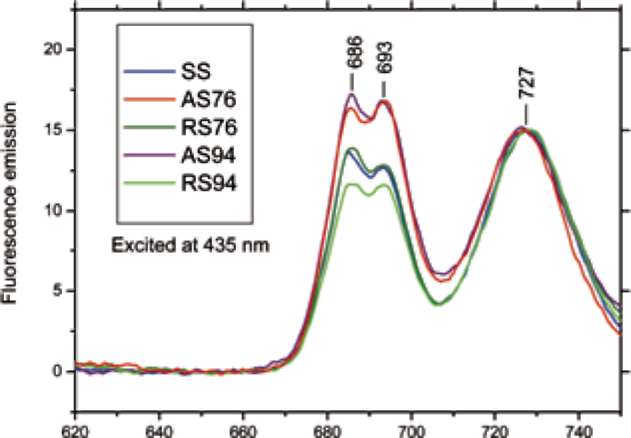
Figure 3 shows 77 K fluorescence emission spectra of chloroplasts from in vitro-grown juvenile (SS), adult (AS76 and AS94), and rejuvenated (RS76 and RS94) S. sempervirens shoots. The emission peak at ∼727 nm originated from photosystem I (Demming and Bjorkman, 1987). The emission peak at ∼685 nm may originate from CP43 and light harvesting complexes, whereas the origin of the emission peak at ∼693 nm originated from func-tional photosystem II (Hung et al., 2010). The relative contents of photosystems II and I were estimated from the amplitudes of fluorescence peaks at ∼693 and ∼727 nm, respectively. Our results in Figure 3 indicate that photo-system II/ photosystem I ratios were significantly higher in the adult (AS76 and AS94) shoots than in the juvenile (SS) and rejuvenated (RS76 and RS94) ones.
|
|
|
|
Figure 2. Photograph of isolated chloroplasts of Sequoia sempervirens shoots by using a ZEISS Axio Imager.Z1 Microscope and PvCam. Red auto-fluorescence of chlorophylls signified the images of chloroplasts.
|
|
|
|
|
|
|
|
Room temperature chlorophyll fluorescence analysis
|
|
|
|
Table 2 shows the maximal quantum efficiency of PSII photochemistry (Fv/Fm) in in vitro-grown juvenile, adult, and rejuvenated S. sempervirens shoots. The Fv/Fm values were almost identical among juvenile (SS), adult (AS76 and AS94) and rejuvenated (RS76 and RS94) shoots. This result confirmed our previous work (Huang et al., 2003b). In addition, this parameter was found to have value around 0.83 under optimal physiological conditions (Demming and Bjorkman, 1987). The Fv/Fm values for in vitro-grown S. sempervirens shoots (Table 1) were very close to this reported optimal value, 0.83, suggesting that all
|
|
|
|
|
|
|
|
Figure 3. 77K-fluorescence emission spectra of chloroplasts from juvenile, adult, and rejuvenated Sequoia sempervirens shoots by exciting chlorophyll at 435 nm (Excitation bandwidth 5 nm; Emission bandwidth 1 nm). Spectra were normalized at 727 nm. All measurements were carried out at 77K, using chloroplast suspensions at a chlorophyll concentration of 50 μg/mL.
|
|
|
|
|
|
|
|
Table 1. Photosynthetic oxygen evolution rates (jimoles 02/mg of Chl x hour) of chloroplasts in juvenile (SS), adult (AS76 and AS94), and rejuvenated (RS and RS94) S. sempervirenes shoots.
|
|
|
|
|
|
|
|
Table 2. Maximal quantum efficiency of photosystem II for juvenile (SS), adult (AS76 and AS94), and rejuvenated (RS76 and RS94) S. sempervirenes shoots.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Values are the mean±SD (n=6 from 3 independent experiments).
|
Values are the mean ± SD (n=6 from 3 independent experiments).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
in the juvenile and the rejuvenated S. sempervirens shoots are correlated with their more vigorous and rapid growth (Huang et al., 2003b). The increased nitrogen enables enhanced protein synthesis; photosynthesis and respiration provide the required energy for rapid growth. In addition, there was no significant difference in maximal quantum efficiency of PSII (Fv/Fm) in shoot tissues of in vitro-grown juvenile, adult, and rejuvenated S. sempervirens (Huang et al., 2003b and Table 2). However, non-photochemical quenching (NPQ) results suggest that a significantly larger fraction of exciting energy was dissipated as heat during the photosynthesis process in AS 76 adult S. sempervirens shoots. Because AS76 adult S. sempervirens shoots have significant lower photosynthetic rates (determined by fresh weight) but are accompanied by normal photosystem II photochemistry (Fv/Fm), the AS76 adult shoots may employ non-photochemical quenching (NPQ) to dissipate excess excitation energy and protect their photosynthetic apparatus from photo-inhibition (Horton and Ruban, 2005). Therefore, these differences in photosynthetic properties and photosystem stoichiometry of juvenile, adult, and rejuvenated shoots may reflect the adjustments of photosynthetic apparatus to acclimate the distinct physiological states of S. sempervirens during phase changes.
|
|
|
|
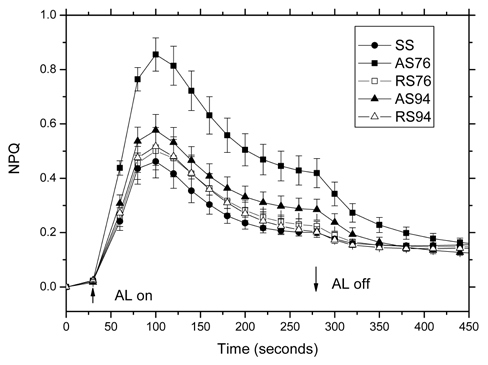
Figure 4. Time-dependent nonphotochemical quenching (NPQ) of juvenile, adult, and rejuvenated Sequoia sempervirens shoots in the presence and absence of CW actinic light (AL). Conditions: Samples were incubated in darkness for 5 min. The intensity of the actinic light was about 80 fimol quanta/(m2+s). Values are the mean ± SE (n=12 - 15).
|
|
|
|
these tissues were in good physiological condition. Figure 4 shows time-dependent non-photochemical quenching (NPQ) of in vitro-grown juvenile, adult, and rejuvenated S. sempervirens shoots in the presence and absence of CW actinic light (AL). NPQ values under actinic light (AL) were apparently higher for AS76 adult tissues compared to juvenile (SS), and rejuvenated (RS76 and RS94) ones. It require further study to determine whether NPQ of AS94 is significantly higher than that of rejuvenated (RS76 and RS94) ones. Our results suggested that thermal dissipation of exciting energy during photosynthesis was significantly enhanced in AS76 adult S. sempervirens shoots.
|
|
|
|
In our recent study, the protein abundance and transcript abundance of a phosphoprotein, SsOEE2, were consistently lower in Sequoia shoots during the adult stage but increased with repeated grafting (Chang et al., 2011). SsOEE2 is one of the extrinsic polypeptides on the lumen side of photosystem II. This correlation between rejuvenation and protein and transcript abundance of the gene suggests that SsOEE2 is associated with rejuvenation (Chang et al., 2011). Further study is required to determine the effect of SsOEE2 phosphorylation on photosystem II function and its role in S. sempervirens rejuvenation.
|
|
|
|
|
|
|
|
|
Acknowledgements. We thank Bau-Lian Huang and Ching-I Kuo for preparations of S. sempervirens materials and for assistance with chloroplast isolations. We also thank Mei-Jane Fang for assistance with microscope photography. Our investigation was supported by the Institute of Plant and Microbial Biology, Academia Sinica.
|
|
|
|
Our results revealed significant differences in the pho-tosynthetic properties and photosystem stoichiometry of S. sempervirens shoots during phase change. Our 77K fluorescence analysis in Figure 3 suggested that photosystem II/photosystem I ratios were significantly higher in adult S. sempervirens shoots (AS76 and AS94). Our results indicated significant alterations of photosystem stoichiometry in chloroplasts of S. sempervirens shoots during phase change. Our previous results showed that photosynthesis rates and overall chlorophyll contents (determined by fresh weight) were lower in adult S. sempervirens shoots (AS76 and AS94) (Huang et al., 2003b). Taken together, we proposed that slightly higher photosynthetic O2 evolution rates in chloroplasts (on the same chlorophyll basis) from adult S. sempervirens shoots (see Table 1) might be due to significantly higher photosystem II/photosystem I ratios in adult S. sempervirens shoots. It still requires future ex-periments to verify our proposal.
|
|
|
|
|
|
|
|
Baker, N.R. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59: 89-113.
Chang, I.-F., P.-J. Chen, C.-H. Shen, T.-J. Hsieh, Y.-W. Hsu, B.-L. Huang, C.-I. Kuo, Y.-T. Chen, H.-A. Chu, K.-W. Yeh, and L.-C. Huang. 2010. Proteomic profiling of proteins associated with the rejuvenation of Sequoia sempervirens (D. Don) Endl. Proteome Sci. 8: 64.
Demming, B. and O. Bjorkman. 1987. Comparison of the effect of excess light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. Planta 171: 171-184.
|
|
|
|
The higher photosynthetic rates, nitrogen content, and respiration rates (determined by fresh weight) observed
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHU et al. ― Phase change and photosynthesis in Sequoia sempervirens
|
|
|
|
|
|
|
|
|
Horton, P. and A.V. Ruban. 2005. Regulation of Photosynthesis under Stress: Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J. Exp. Bot. 56: 365-373.
Huang, H.-J., Y. Chen, J.-L. Kuo, T.-T. Kuo, C.-C. Tzeng, B.-L.
Huang, C.-M. Chen, and L.-C. Huang. 1996. Rejuvenation of Sequoia sempervirens in vitro: changes in isoesterases and isoperoxidases. Plant Cell Physiol. 37: 77-80.
Huang, L.-C., S. Lius, B.-L. Huang, T. Murashige, E.F.M. Mah-
di, and R. Van Gundy. 1992. Rejuvenation of Sequoia sem-pervirens by repeating grafting of shoot tips onto juvenile rootstocks in vitro. Plant Physiol. 98: 166-173.
Huang, L.-C., L.-Y. Lin, C.-M. Chen, L.-J. Chen, B.-L. Huang,
and T. Murashige. 1995. Phase reversal in Sequoia semper-
virens in relation to mtDNA. Physiol. Plant. 94: 379-383. Huang, L.-C., S.-. Pu, T. Murashige, S.-F. Fu, T.-T. Kuo, D.-D.
Huang, and H.-J. Huang. 2003a. Phase and age-related differences in protein tyrosine phosphorylation in Sequoia sempervirens. Biol. Plant. 47: 601-603.
|
Huang, L.-C., J.-H. Weng, C.-H. Wang, C.-I. Kuo, and Y.-J.
Shieh. 2003b. Photosynthetic potentials of in vitro-growth juvenile, adult, and rejuvenated Sequoia sempervirens (D.
Don) Endl. Shoots. Bot. Bull. Acad. Sin. 44: 31-35. Kuo, J.-L., H.-J. Huang, C.-M. Cheng, L.-J. Chen, B.-L. Huang,
L.-C. Huang, and T.-T. Kuo. 1995. Rejuvenation in vitro: modulation of protein phosphorylation in Sequoia semper-
virens. J. Plant Physiol. 146: 333-336. Hung, C.-H., H.-J. Hwang, Y.-H. Chen, Y.-F. Chiu, S.-C. Ke,
R.L. Burnap, and H.-A. Chu. 2010. Spectroscopic and functional characterizations of Synechocystsis 6803 mutant on and near the heme axial-ligand of cytochrome B559 in pho-tosystem II. J. Biol. Chem. 285: 5653-5663.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol.
Plant. 15: 473-497.
|
|
|
|
|
|
|
|
紅杉試管幼年、老齡、及其復幼植莖光合作用特性 和光系統比例改變之研究
|
|
|
|
|
|
|
|
朱修安1 張英峯2 沈金輝2 陳玉婷3 王幸婷1 黃麗春1 葉開溫2
|
|
|
|
|
|
|
|
1中央研究院植物暨微生物學研究所
2國立台灣大學植物科學研究所
3國立中興大學基因體暨生物資訊學研究所
|
|
|
|
|
|
|
|
本研究主要探討紅杉不同的生長相(幼年期、成齡期、及返老還輕微後)的植莖光合作用特性和光
系統比例的變化情形。發現成齢老株的葉綠體中的光系統二對應光系統一的比例和光合作用釋放氧氣的
速率明顯的高於幼年莖和復幼莖。顯示紅杉的葉綠體中光系統的比例在不同的生長相有顯著的變化。
雖然幼年期或成齢期的光合系統的電子傳遞效率 (Fm-Fo)/Fm並無明顯的差異,但是成齢老株AS76的光
合作用有明顯較高的熱消散(Nonphotochemical quenching; NPQ)現象。這些光合作用特性和光系統比例
的變化可能反映了紅杉不同的生長相間的生理狀態的差異。
|
|
|
|
|
|
|
|
關鍵詞:紅杉;生長相;光合作用;光系統比例;熱消散。
|
|
|
|
|
|
|
|
|
|
|
|