232
Botanical Studies, Vol. 53, 2012
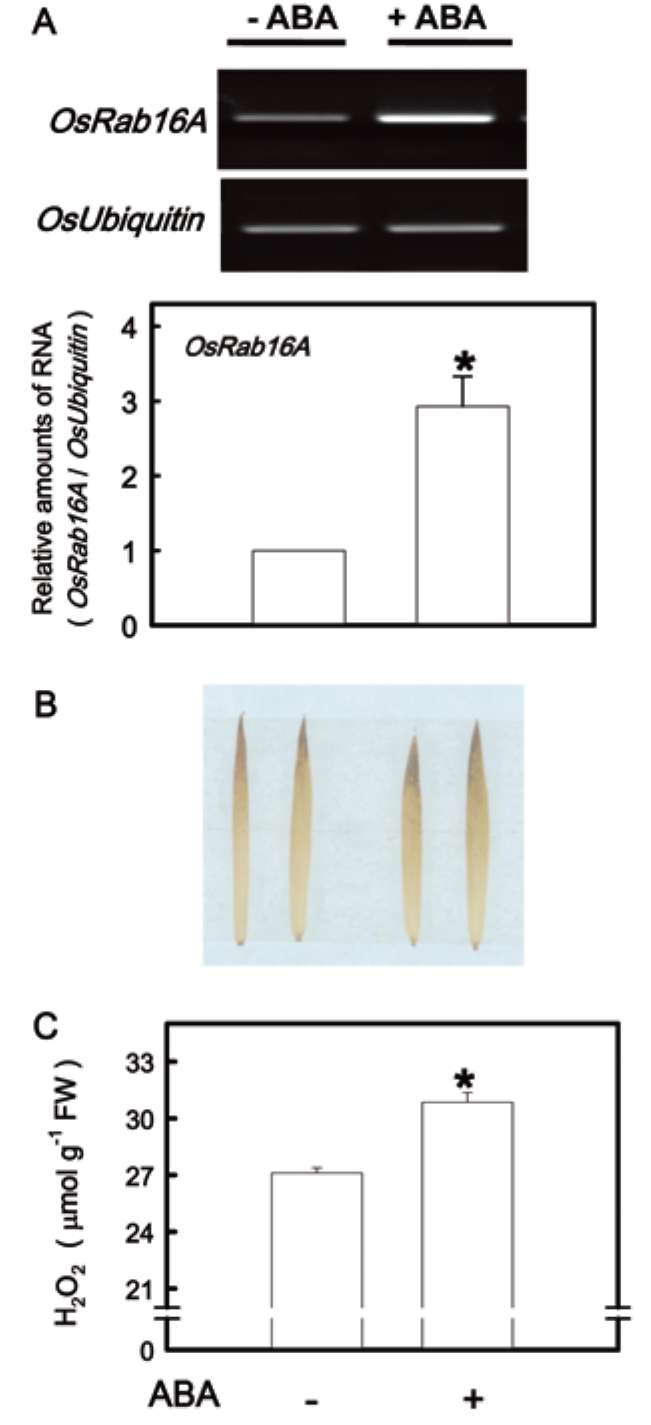
suspension cells on treatment with ABA (Mundy and Chua, 1988; Hong et al., 2009). Thus, ABA content in this study was judged by the transcripts of OsRab16A. Figure 3A shows that the expression of OsRab16A was increased in K-deficient leaves. When ABA content was determined by the ELISA, we also found that K deficiency resulted in an increase in ABA content in the second leaves (Figure 3B).
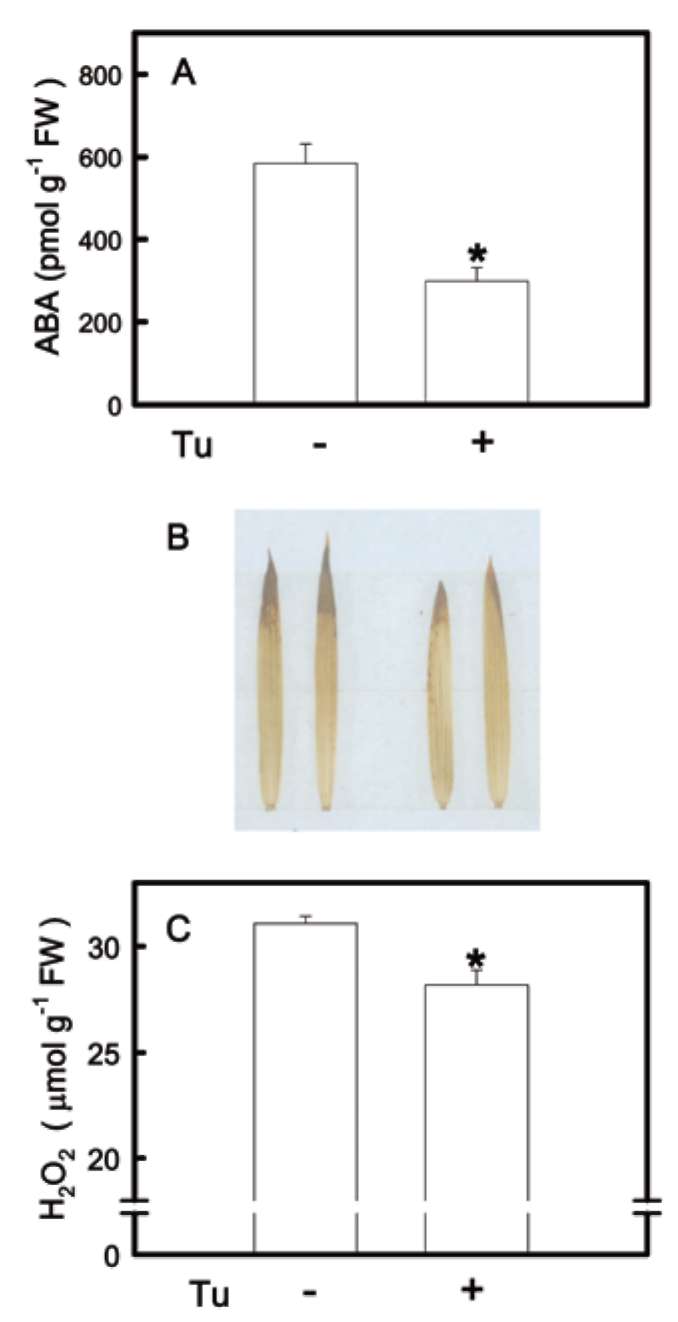
ABA accumulation is not induced by H2O2
To examine if ABA accumulation induced by K defi-
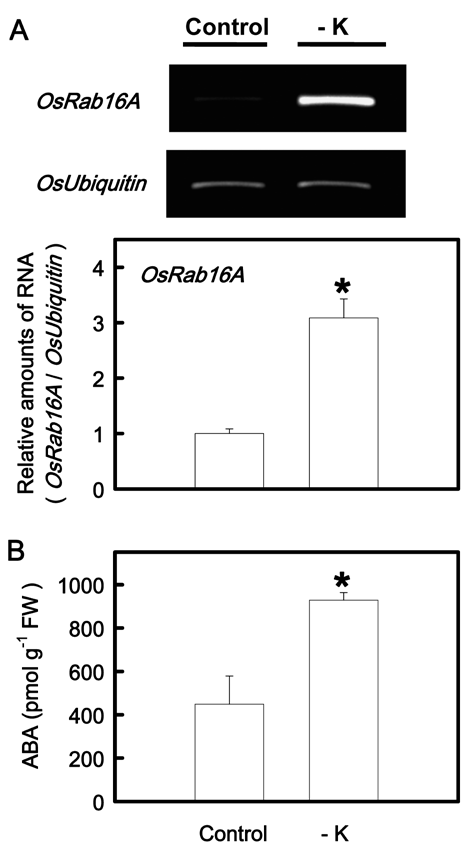
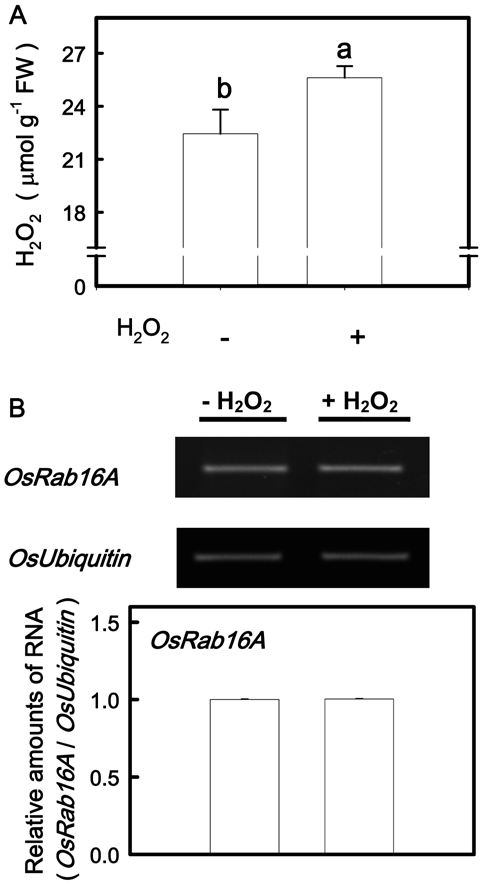
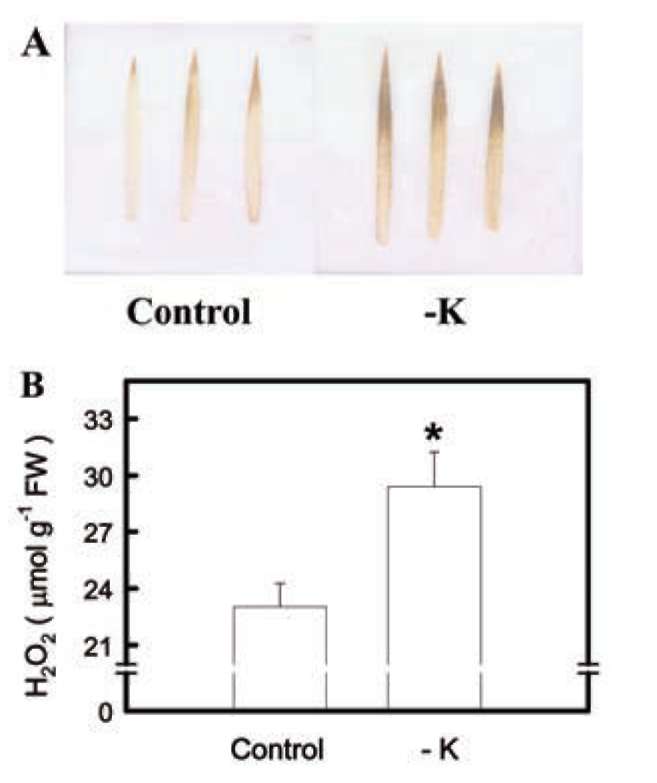
Figure 1. DAB-H2O2 reaction product (A) and H2O2 content (B) in the second leaves of rice seedlings grown under K-sufficient (control) and -deficient (-K) conditions for 12 days. Bars show means ± SE (n = 4). Asterisk represents values that are significantly different between control and -K treatments at P<0.05.
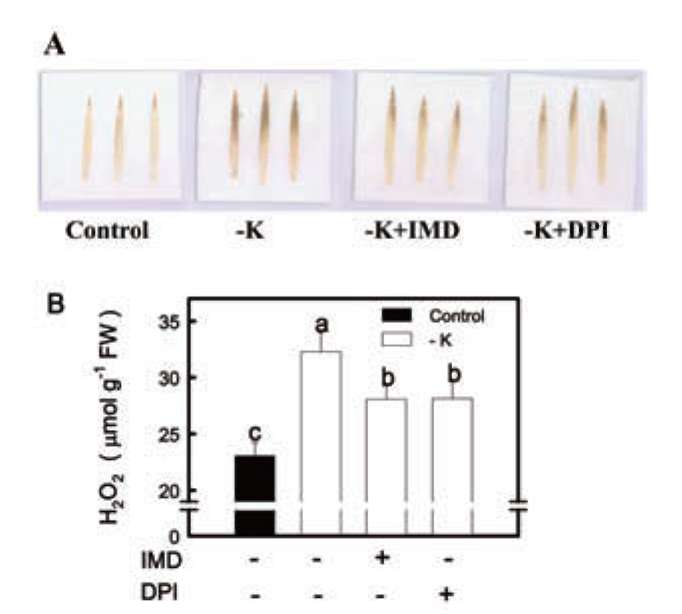
Figure 3. The mRNA level of OsRab16A (A) and the content of ABA (B) in the second leaves of rice seedlings grown under K-sufficient (control) and -deficient (-K) conditions for 12 days. The value of OsRab16A gene was adjusted by a corresponding amount of OsUbiquitin. After the adjustment by OsUbiquitin, the reaction of the control was treated as the normalized reference, with a value of one, for determining the relative amount of mRNA of OsRab16A gene. Bars show means ± SE (n = 3 for OsRab16A transcripts and n = 4 for ABA content). Asterisks represent values that are significantly different between control and -K treatments at P<0.05.
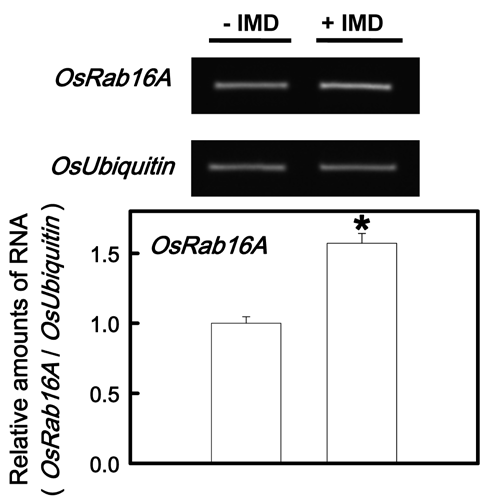
Figure 2. Effect of IMD and DPI on DAB-H2O2reaction product (A) and H2O2 content (B) in the second leaves of rice seedlings grown under K-deficient (-K) conditions. Rice seedlings were first grown under -K conditions for 12 days then transferred to -K nutrient solution with or without 100 μM IMD and 50 μM DPI for another 3 h. Bars show means ± SE (n = 4). Values with the same letter are not significantly different at P<0.05.