|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies (2012) 53: 239-242.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pseudallin, a new antibiotic produced by the human pathogenic fungus Pseudallescheria boydii, with ecological significance
|
|
|
|
|
|
|
|
Huey-Jen SU1, Mei-Ju LIN2, Yi-Jung TSOU2, and Wen-Hsiung KO2 *
|
|
|
|
|
|
|
|
1Department of Nursing, Meiho University, Neipu, Pingtung, Taiwan
2Department of Plant Pathology, National Chung Hsing University, Taichung, Taiwan
|
|
|
|
|
|
|
|
(Received July 20, 2011; Accepted October 7, 2011)
|
|
|
|
|
|
|
|
ABSTRACT. Pseudallescheria boydii is an emerging fungal human pathogen causing serious disease in both immunocompromised and immunocompetent patients. Recent report showed that this fungus is capable of controlling black leaf spot of cabbage caused by Alternaria brassicicola by producing a fungistatic substance strongly inhibitory to fungi. Production of the inhibitory substance by P. boydii during colonization of plant tissues in soil appears to account for its strong competitive saprophytic ability and widespread occurrence in soil. In this study, freeze-dried powder of liquid culture of P. boydii was extracted with ethanol and fractionated on silica gel column to give four fractions. The most active fraction was further purified by silica gel chromatography. The resulting compound 1 was structurally characterized as 6-6'-bis (2HH-pyran-3-carbalde-hyde) ether which is a new natural product showing strong inhibitory activity against A. brassicicola. The new antibiotic, a yellow oil with a molecular weight of 234, was named pseudallin. To our knowledge, this is the first report of antibiotic production by a non-dermatophyte human pathogenic fungus. Results from this study indicate the importance of antibiotic production in competitive saprophytic colonization of plant debris by P. boydii in soil, which in turn may explain its widespread occurrence.
|
|
|
|
|
|
|
|
Keywords: Alternaria brassicicola; Antibiotic; Black leaf spot; Human pathogen; Plant disease control; Pseu-dallescheria boydii; Pseudallin.
|
|
|
|
|
|
|
|
|
isolate TKF-4 of P. boydii was grown in liquid medium prepared from the same vegetable tissues, the resulting culture was strongly inhibitory to Alternaria brassicicola and was very effective in controlling black leaf spot of cabbage caused by this plant pathogen. This suggests that P. boydii may be able to produce an antibiotic substance to suppress competitors during its colonization of plant tissues in soil, which may account for its strong competitive saprophytic ability in soil (Ko et al., 2010).
|
|
|
|
Pseudallescheria boydii is an emerging fungal pathogen causing localized as well as disseminated infections on both immunocompromised and immunocompetent patients (Thornton, 2009). The problems resulted from this pathogen include allergic bronchopulmonary disease, chronic lung lesions, fatal central nervous system infections and pneumonia The fungus is distributed worldwide in soils. It was isolated from agricultural, forest and garden soils (Rainer and de Hoog, 2006), and found even in soils of potted plants in hospitals (Summerbell et al., 1989) and oil-soaked soils (April et al., 1998). It is intriguing why this human fungal pathogen is so widespread in soils.
|
|
|
|
The objective of this study is to isolate and purify this antibiotic substance in large quantity, and to use the purified compound for determination of its molecular structure. Details of the study are reported herein.
|
|
|
|
|
|
|
|
Recently, microorganisms with ability to use vegetable tissues for growth in natural soil were selectively isolated. Among them were several isolates of P. boydii indicating their possession of a strong competitive saprophytic ability, which in turn may explain the widespread occurrence of this human pathogen in soil (Ko et al., 2010). When
|
|
|
|
|
Sources of fungal isolates used
|
|
|
|
Pseudallescheria boydii TKF-4 was isolated from a farm soil in central Taiwan (Ko et al., 2010). A. brassici-cola Aba-31 isolated from Chinese cabbage was provided by Dr. J. W. Huang, Department of Plant Pathology, National Chung Hsing University, Taichung, Taiwan. Both fungi were maintained on 10% V8 agar containing 10% V8 juice, 0.02% CaCO3 and 2% agar (Wang et al., 2010).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
Production and extraction of antibiotic substance
|
|
|
|
|
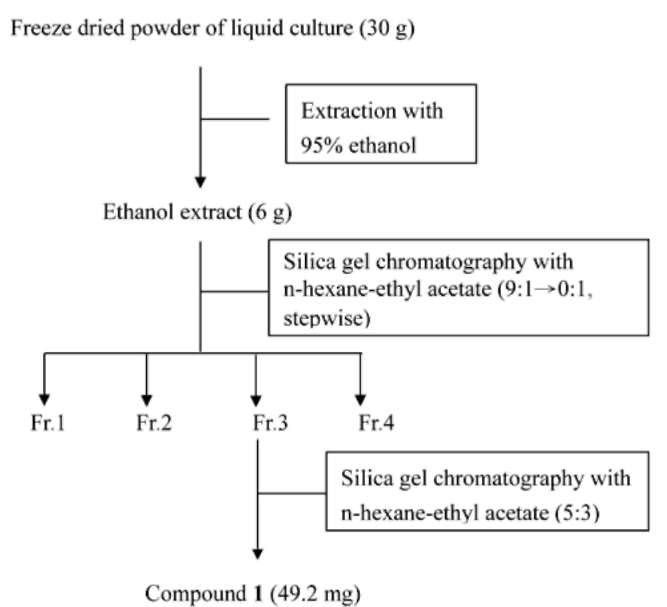
A 50-mL aliquot of vegetable broth prepared as previously described (Ko et al., 2010, 2011) was dispensed in a 250-mL flask. After autoclaving, each flask was inoculated with a culture block (ca. 4 mm x 5 mm x 3 mm) of P. boydii TKF-4 grown on 10% V8 agar. After incubation on a shaker for 2 weeks, the culture was ground in an Omni mixer at 4,000 rpm for 1 min and freeze-dried. The freeze-dried powder was extracted with 95% ethanol. The ethanol extract was then chromatographed on a silica gel column (230-400 mesh, 2 cm x 60 cm) using a gradient elution of n-hexane-ethyl acetate (9:1 ― 0:1) to afford four fractions. For each fraction, the organic solvent was evaporated and the resulting aqueous portion was bioassayed. The most active fraction was further chromatographed on a silica gel column (1.5 cm x 60 cm) (Figure 1). Silica gel 60 (230-400 mesh; Merck, Darmstadt, Germany) was used for column chromatography.
|
|
|
|
Figure 1. Schematic diagram of extraction and fractionation of liquid culture of Pseudallescheria boydii.
|
|
|
|
|
|
|
|
|
|
|
|
To test the antibiotic activity of the fractions of culture extract, conidia of A. brassicicola Aba-31 were produced by growing the fungus on 10% V8 agar at 24°C under light for 6 days. The conidial suspension was prepared by agitating two pieces of culture blocks (ca. 5 mm x 5 mm x 3 mm) in 5 mL sterile distilled water in a test tube with a Vortex mixer for 30 s. The concentration of conidia was adjusted to 2 x 104 spores mL-1 with a Pipetman microli-ter pipette (West Coast Scientific, Oakland, CA) (Ann et al., 2010). The concentrations of 100, 500 and 1000 ppm were used for each extract fraction. A 10-[iL aliquot of the extract was mixed with 10 [iL of conidial suspension in a cavity of a sterile eight-cavity slide. Each slide with spores was kept moist by placing on an L-shaped glass rod in a 9-cm Petri plate containing 10 mL sterile distilled water. Germination was recorded after incubation at 24°C for 5 h, and 100 spores were counted in each of the three replicates.
|
fractions was assayed by testing their ability to inhibit conidial germination of A. brassicicola. At 1000 ppm, fractions 2 and 3 inhibited germination of A. brassicicola conidia completely (Table 1). Fraction 3 was still strongly inhibitory to spore germination at 500 and 100 ppm, reducing the germination from 96% in the control to 15 and 39%, respectively. Fraction 2 was only moderately inhibitory to the spore germination at 500 ppm, and became non-inhibitory at 100 ppm. Fractions 1 and 4 were only slightly inhibitory even at 1000 ppm. Fraction 3, therefore, was further fractionated by chromatography on silica gel column. The initial 250 mL fraction was collected and concentrated using a vacuum evaporator to give 49.2 mg compound 1 as yellow oil. Compound 1 was strongly inhibitory to A. brassicicola, completely suppressing its spore germination at 1000 ppm. Even at 50 ppm, it was still inhibitory to the germination (data not shown).
|
|
|
|
|
|
|
|
|
The HR-ESI-MS of compound 1 indicated a molecular ion peak at m/z 257.0425, which corresponded to a molecular C12H10O5Na. IR absorptions were indicative of C=O group (1738 cm-1), a C=C double bond (1648 cm-1), and a C-O bond (1192 cm-1). The 1H-NMR spectrum of com-
|
|
|
|
For analysis of compound 1, the UV spectra were obtained on a Helios Omega UV-Visible spectrophotometer (Thermo Scientific, Japan). IR spectra were recorded on a Jasco FT-IR-5300 infrared spectrophotometer (Japan). All of the NMR spectra were recorded on a Varian Unity INOVA 500 FT-NMR (USA) instrument. ESI-MS and HR-ESI-MS data were recorded on a BRUKER APEX II mass spectrometer (Germany).
|
|
|
|
Table 1. Activity of fractions 1 to 4 of culture extract of Pseudallescheria boydii from silica gel chromatography, against conidial germination of Alternaria brassicicola.
|
|
|
|
|
|
|
|
|
|
|
|
A total of 1500 mL of liquid culture of P. boydii was freeze-dried to give 30 g of powder. Extraction of the powder with 95% ethanol resulted in 6 g of extract. Chro-matography of the extract on silica gel column yielded four fractions (Figure 1). The antibiotic activity of these
|
|
|
|
aGermination in water control was 96%.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SU et al. ― New antibiotic produced by Pseudallescheria boydii
|
|
|
|
|
|
|
|
|
|
Although production of antibiotics by dermatophyte fungi has long been known (Youssef et al., 1978), to our knowledge this is the first report of antibiotic production by a non-dermatophyte human pathogenic fungus. The new antibiotic pseudallin is a bis compound. Its monomer 6-hydroxy-2//-pyran-3-carbaldehyde has been isolated from the medicinal plant Crinum yemense. This pyran derivative is a tyrosinase inhibitor which could be used as a skin whitening agent (Abdel-Halim et al., 2008). Whether pseudallin also has similar tyrosinase inhibiting activity remains to be investigated.
|
|
|
|
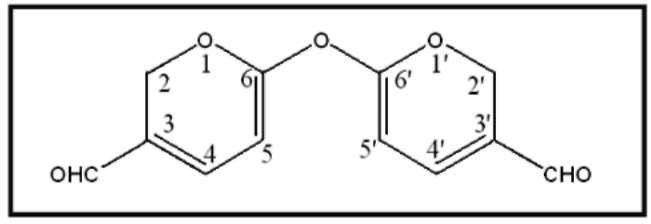
Figure 2. Structure of compound 1 from the liquid culture of
Pseudallescheria boydii.
|
|
|
|
pound 1 showed a methylene proton signal at 5 4.64 (2H, br s), two aromatic proton signals at δ 6.46 (1H, d, J = 3.5Hz) and 7.18 (1H, d , J = 3.5Hz), and an aldehydic proton at 5 9.48 (1H, s). The 13C-NMR and DEPT spectrum of compound 1 showed an oxygen-bearing secondary carbon at 5 57.1, two tertiary carbon, one carbonyl carbon at 5 177.8, and two quaternary carbon. Based on above data, the compound 1 was structurally characterized as a symmetric compound .The NOESY revealed a cross-peak each of H-2/H-4, and H-5/CHO. The HMBC showed correla-tion of H-2/ C-4, -6, H-4/ C-2, -3, -5, -6, H-5/ C-3, -4, -6, CHO, and C//O/C-3. Based on these results and the corre-sponding data of 6-Hydroxy-2H-pyran-3-carbaldehyde and pyran carbaldehyde derivatives (Schult et al., 1972; Abdel-Halimet et al., 2008), the compound 1 was confirmed as 6-6'-bis(2//-pyran-3-carbaldehyde) ether (Figure 2) which is a new natural product and named pseudallin.
|
|
|
|
Although pseudallin is very effective in controlling black leaf spot of cabbage caused by A. brassicicola in the greenhouse (Ko et al., 2010), its potential of being developed into a commercial product is far from certain. Further evaluation of its disease control ability in the field and its toxicity to the non-target organisms in nature should be carried out to decide if it could be used effectively and safely as a plant disease control agent. It would also be interesting to know if the monomer of pseudallin from C. yemense also has the fungistatic activity.
|
|
|
|
The chemical structure of pseudallin shows neither positive nor negative charge on its molecule. This explains why the inhibitory activity of the culture extract of P. boydii was not affected by shaking with cation or anion exchange resins (Ko et al., 2010). The molecular weight of pseudallin is 234. However, only a small amount of the inhibitory substances in the culture extract passed through the membrane tubing with molecular weight cut-off of 500 (Ko et al., 2010). This led to the misinterpretation that the molecular weight of the inhibitor was larger than 500. It is conceivable that most of the inhibitory substances were stuck on or embedded in the impurities, making them difficult to pass through that membrane tubing. Certain components of the impurities in the culture extract may also serve as a surfactant, which in turn may explain why the oily pseudallin in the freeze dried powder was extractable with water (Ko et al., 2010).
|
|
|
|
Molecular structure analysis data
|
|
|
|
6-6'-bis(2//-pyran-3-carbaldehyde) ether (compound 1): Yellow oil; UV λmax (MeOH) nm (log ε) 290 (2.30), 247 (3.35); IR (neat) νmax1738, 1648, and 1192 cm-1; 1H-NMR (CDCI3, 500 MHz) 5 4.64 (2 H, br s, H-2), 6.46 (1 H, d, J = 3.5 Hz, H-4), 7.18 (1 H, d, J = 3.5 Hz, H-5), 9.48 (1 H, s, H of aldehyde); 13C NMR(CDCl3, 125 MHz) 5 57.1 (C-2), 152.0 (C-3), 109.9 (C-4), 123.5 (C-5), 161.1 (C-6), 177.8 (CHO). ESIMS m/z 257 [M+Na]+; HR-ESI-MS m/z 257.0425 (calcd for CnH10O5Na [M+Na]+, 257.0426).
|
|
|
|
|
|
|
|
|
Acknowledgments. We thank Dr. J. W. Huang for the culture of A. brassicicola. This research was supported in part by grants from Council of Agriculture and National Science Council of Taiwan (NSC99-2811-B-005-002).
|
|
|
|
A previous study demonstrated the ability of P. boydii to colonize vegetable tissues for multiplication in soil (Ko et al., 2010). Since soil contains abundant and diverse microorganisms (Alexander, 1977), the study indicates that the fungus has strong ability to compete with other microorganisms to obtain nutrients under such environment (Garrett, 1970). In this study, it was found that P. boydii was able to produce pseudallin, a new antibiotic, when grown in the broth prepared from the same vegetable tissues as those used in the previous study (Ko et al., 2010). It is likely that during colonization of plant debris in soil, the fungus is able to produce pseudallin to inhibit other competitors. This may account for its strong competitive saprophytic ability (Garrett, 1970), which in turn may explain the widespread occurrence of this human pathogenic fungus in soil.
|
|
|
|
|
|
|
|
Abdel-Halim, O.B., A.M. Marzouk, R. Mothana, and N. Awadh. 2008. A new tyrosinase inhibitor from Crinum yemense as potential treatment for hyperpigmentation. Pharmazie 63: 405-408.
Alexander, M. 1977. Introduction to Soil Microbiology, 2nd edn. John Wiley & Sons, New York.
Ann, P.J., J.N. Tsai, T.C. Wang, C.H. Chen, M.J. Lin, and W.H.
Ko. 2010. Reevaluation of the report of A2 mating type of Phytophthora infestens on tomato in Taiwan. Bot. Stud. 51: 203-207.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
April, T.M., S.P. Abbott, J.M. Foght, and R.S. Currah. 1998.
Degradation of hydrocarbons in crude oil by the ascomycete Pseudallescheria boydii (Microascaceae). Can. J. Micro-biol. 44: 270-278.
Garrett, S.D. 1970. Pathogenic Root-Infection Fungi. Cambridge University Press, London.
Ko, W.H., Y.J. Tsou, Y.M. Ju, H.M. Hsieh, and P.J. Ann. 2010.
Production of a fungistatic substance by Pseudallescheria boydii isolates and its significance. Mycopathologia 169: 125-131.
Ko, W.H., C.H. Yang, M.J. Lin, C.Y. Chen, and Y丄Tsou. 2011. Humicola phialophoroides sp. nov. from soil with potential for biological control of plant diseases. Bot. Stud. 52: 197202.
Rainer, J. and G.S. de Hoog. 2006. Molecular taxonomy and colony of Pseudallescheria, Petriella and Scedosporium polificans (Microascaceae) containing opportunistic agents
|
on humans. Mycol. Res. 110: 151-160.
Schulte, K.E., G. Ruucker, and M. El-Kersch. 1972. Nicotin und 3-formyl-4-hydroxy-2H-pyran aus Herpestis monniera.
Phytochemistry 11: 2649-2651.
Summerbell, R.C., S. Krajden, and J. Kane. 1989. Potted plants in hospitals as reservoirs of pathogenic fungi. Mycopatholo-gia 106: 13-22.
Thornton, C.R. 2009. Tracking the emerging human pathogen Pseudallescheria boydii by using highly specific monoclonal antibodies. Clin. Vaccine Immunol. 16: 756-764.
Wang, P.H., Y.S. Chen, M.J. Lin, Y.J. Tsou, and W.H. Ko. 2010. Severe decline of wax apple trees caused by Fusarium so-lani in northern Taiwan. Bot. Stud. 51: 75-80.
Youssef, N., C.H.E. Wyborn, and G. Holt. 1978. Antibiotic production by dermatophyte fungi. J. Gen. Microbiol. 105: 105-111.
|
|
|
|
|
|
|
|
Pseudallin ,由人體病原真菌Pseudallescheria boydii戶斤產生
具有生態重要性的新抗生素
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pseudallescheria boydii是一個新興的人體病原真菌,引起免疫功能低及免疫功能正常的病患發生嚴
重疾病。最近的硏究結果顯示此真菌能產生抑制物質,能防治由Alternaria brassicicola造成的甘藍黑斑
病,當它纏據在土壤中的植物組織會產生抑制物質,故具有很強的腐生能力並廣泛的存在土壤之中。本
硏究中,將P. boydii培養液冷凍乾燥,以酒精進行萃取後,以矽膠管柱進行層析,分離出四個沖提物,
再以矽膠管柱層析法純化具活性之沖提物。1號化合物的結構鑑定爲6-6'-bis(2H-pyran-3-carbaldehyde)
ether,爲一天然的物質'具強抑制A. brassicicola '這個新的抗生素爲黃色油狀物'分子量爲234 ,命名
爲Pseudallin 。就我們所知,這是第一個報導由非皮膚癬菌之人體病原真菌產生的抗生素。這個硏究結
果顯示P. boydii產生的抗生素的重要性,抗生素的產生提供該菌在土壤植物殘體中腐生纏據的競爭力,
也可以解釋它普遍存在的原因。
|
|
|
|
|
|
|
|
關鍵詞:十字花科蔬菜黑斑病菌;抗生素;黑斑病;人體病原菌;植物病害防治;波氏假性黴樣菌;假
性黴樣素。
|
|
|
|
|
|
|
|
|
|
|