SHEUE et al. ― Stipules and colleters of the mangrove Rhizophoraceae
245
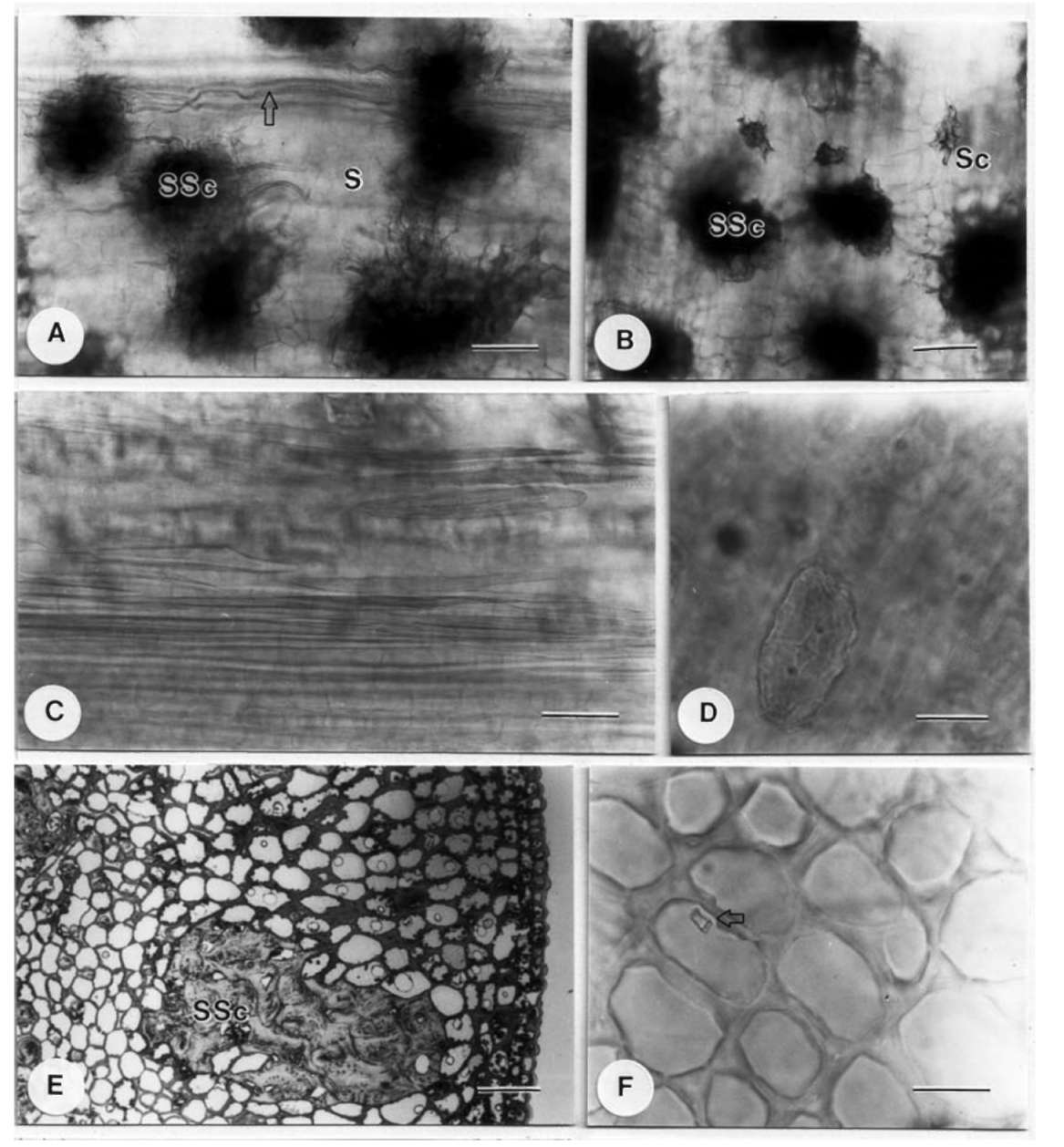
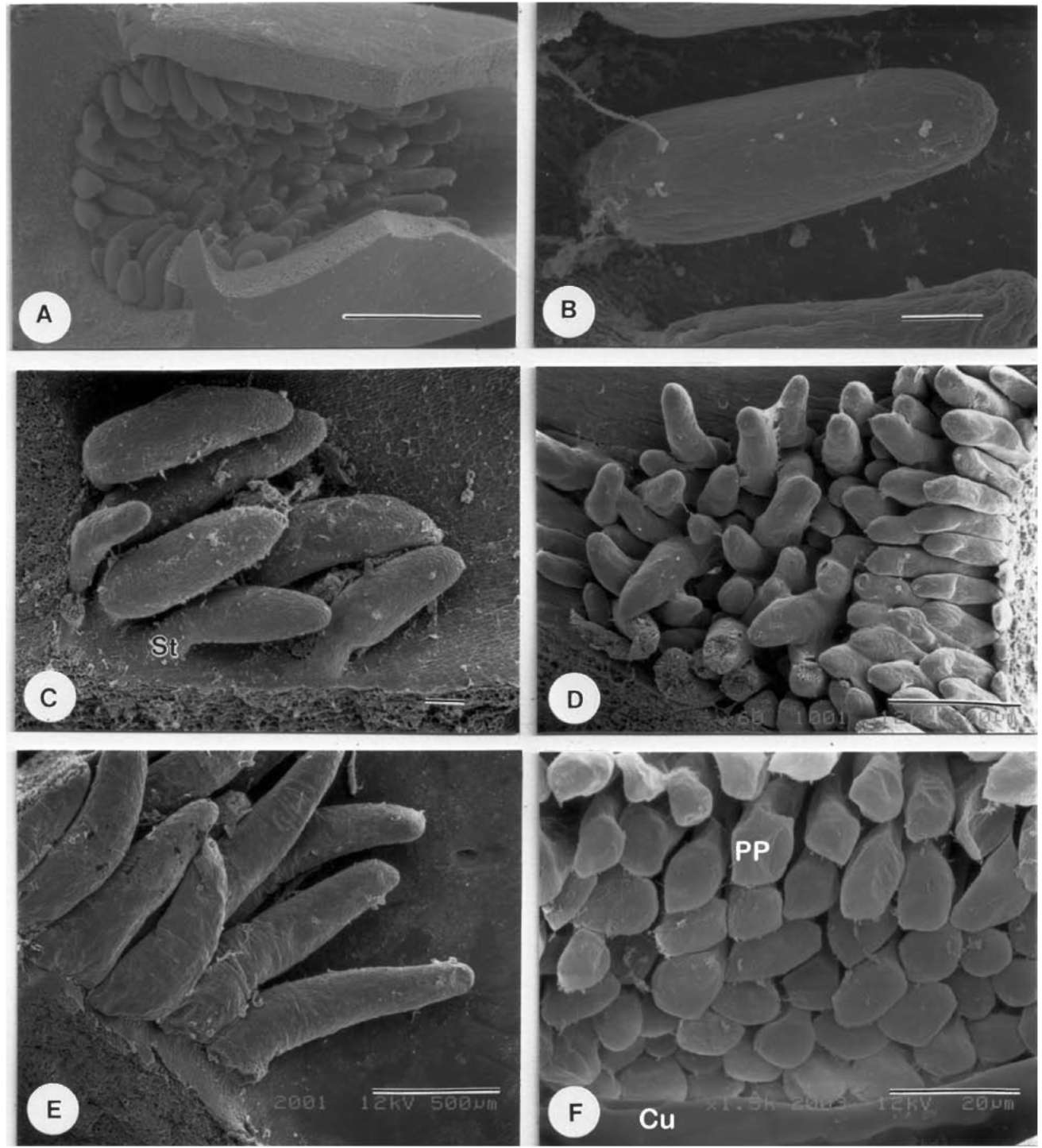
scope (Hitachi, Tokyo, Japan), after treating with ethanol series dehydration, critical point drying and gold coating, allowing shape and size determination in particular. These stipules were also treated with the clearing method (Chiang, 1990) to investigate features such as sclereid id-iobalsts and crystals.

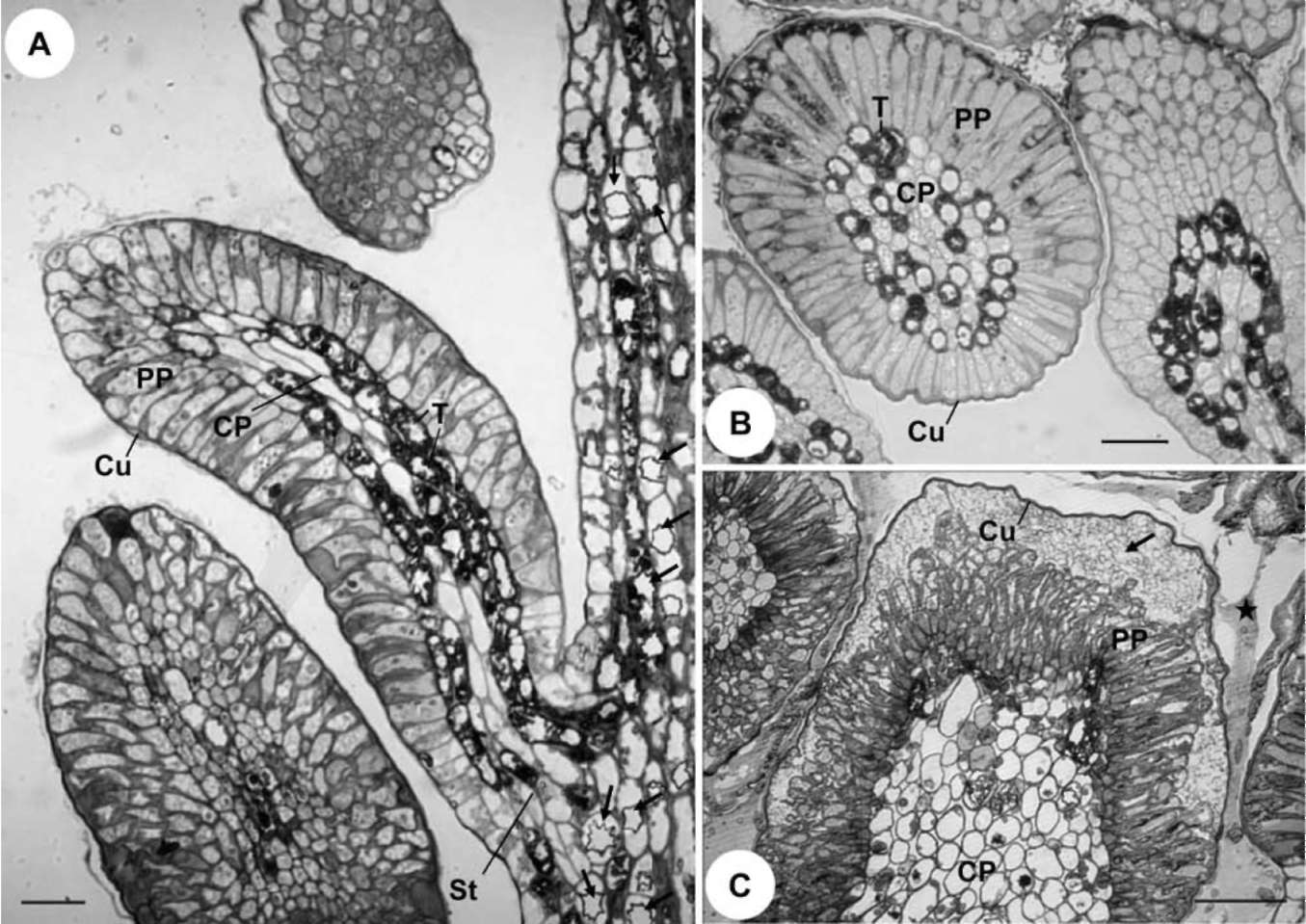
For field fixation for anatomical study, the base of each stipule with colleters was cut into several small pieces and put into 1.25-1.5% glutaraldehyde in 0.1 M phosphate buffer with 5% sucrose. Materials were stored in this fixative for 5-7 days during collection abroad. For the second fixation, materials were subsequently transferred to 1% OsO4 in 0.1 M phosphate buffer for about 4 hours. After dehydration through an ethanol series, materials were infiltrated for 3 days and embedded within Spurr's-resin. The embedded materials were polymerized in an oven at 70°C for 12 hours. Semi-thin sections (1 μm) were made by an Ul-tracut E (Leica, Wetzlar, Germany) or a MTX Microtome (RMC, Tucson, USA), stained with 0.1% Toluidine blue for 1 minute or so, and then observed and photographed with a BH-2 Light Microscope (Olympus, Tokyo, Japan).
Figure 1. The mangrove Rhizophoraceae with shoots and stipules. (A) The shoot apices and top nodes of Ceriops australis with stipules (broad arrows), which are interpetiolar and caducous. Primodia enclosed by the stipules are always bathed by a viscous fluid. Young expanded leaves coated with resin-like mucilage (thin arrow); (B) A fresh stipule of Bruguiera exaristata showing yellowish aggregated finger-like colleters with viscous exudation at the adaxial base. Scale bar = 1 mm.
Table 2. Morphological and structural characters of stipules and colleters of the mangrove Rhizophoraceae, including aggregated form, number of rows and total number of colleters in each stipule, and individual shape and color of colleters.
Taxon
|
Stipule
|
Colleter
|
|||
Morphology/ length (cm)/ Sc
|
Aggregated form/ row no./ total no.
|
Shape/ color
|
|||
|
Bruguiera cylindrica
|
Round/ 3.0-4.0/ no Sc
|
1晨/ 4-7/ 70-90
|
SRs/ MW
|
||
|
Bruguiera exaristata
|
Round/ 2.0-3.0/ no Sc
|
晨/ 9-14/ 120-135
|
SRs/ MW-MY
|
||
|
Bruguiera hainesii
|
Round/ 3.5-4.2/ no Sc
|
晨/ 12-15/ 100-146
|
SRs/ MW
|
||
|
Bruguiera gymnorhiza
|
Round/ 4.0-5.0/ no Sc
|
1晨/ 9-13/ 200-220
|
SRs/ MW
|
||
|
Bruguiera parviflora
|
Round/ 4.5-6.5/ no Sc
|
國晨/ 6-8/ 160-170
|
SRs/ MW
|
||
|
Bruguiera sexangula
|
Round/ 4.5-5.5/ no Sc
|
晨•/ 3-4 / 35-40
|
SRs/ MW
|
||
|
Ceriops australis
|
Flattened/ 1.0-1.2/ Sc 2 types
|
▲ /9-11/ 90-140
|
SRl/ MW
|
||
|
Ceriops decandra
|
Flattened/ 1.2-2.4/ Sc 2 types
|
▲ /7-8/ 50-70
|
SRl/ MW
|
||
|
Ceriops pseudodecandra
|
Flattened/ 2.0-3.0/ Sc 2 types
|
▲ /8-12/ 80-100
|
SRl/ MW
|
||
|
Ceriops tagal
|
Flattened/ 1.5-2.8/ Sc 2 types
|
▲ /24-26/ 165-205
|
SRl/ MW
|
||
|
Ceriops zippeliana
|
Flattened/ 2.5-3.6/ Sc 2 types
|
▲ / 18-20/ 280-310
|
SRl/ MW
|
||
|
Kandelia candel
|
Flattened/ 3.0-4.0/ no Sc
|
▲ /6-7/ 75-96
|
SRl/ MY
|
||
|
Kandelia obovata
|
Flattened/ 2.5-3.2/ no Sc
|
▲ /8-9/ 95-110
|
SRl/ MY
|
||
|
Rhizophora apiculata
|
Round/ 5.0-8.5/ Sc 3 types
|
―/ 2-4/ 160-200
|
ARse/ Y
|
||
|
Rhizophora mucronata
|
Round/ 4.5-6.5/ Sc 3 types
|
―/ 6-9/ 460-580
|
ARse/ MY
|
||
|
Rhizophora stylosa
|
Round/ 4.5-5.5/ Sc 3 types
|
―/ 4- 6/ 180-300
|
ARse/ MY
|
||
|
Rhizophora x annamalayana
|
Round/ 4.5-6.0/ Sc 3 types
|
―/6-7/130-160
|
ARse/ MY
|
||
|
Rhizophora x lamarckii
|
Round/ 4.5-5.5/ Sc 3 types
|
― / 2-3/ 75-90
|
ARse/ MY
|
||
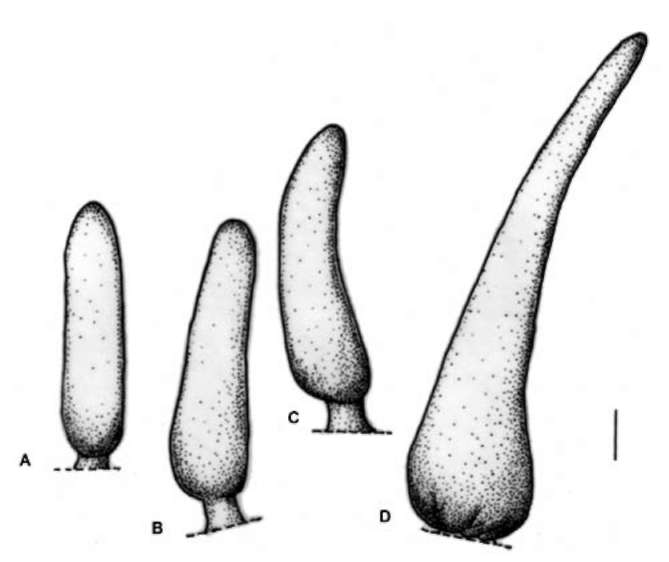
Symbols: aggregated form: broad band —; narrow band —; rectangle I; trapezoid A; semicircle triangle Abbreviations: ARse-Sessile acuminate rod; MW-milky white; MY-milky yellow; Y-yellow; Sc: sclereid idioblast; SRl-long stalked rod; SRs-short stalked rod.