294
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
||
|
|
|
|
|||
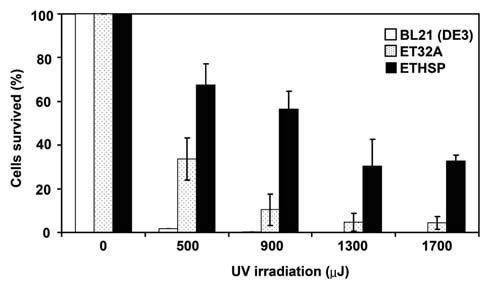
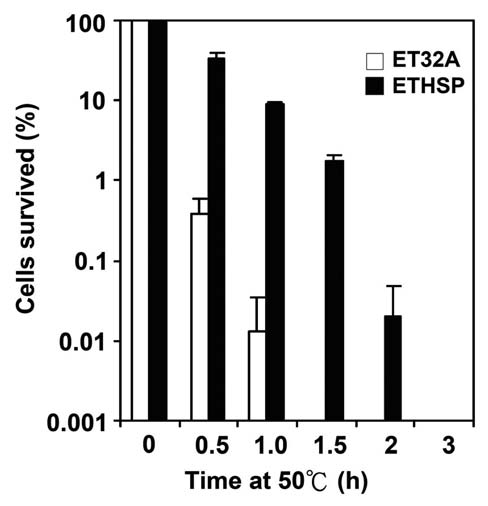
cells was 33%. The difference in survival rates at 50°C for 0.5 h was greater than 80 folds. Even after 1 h exposure to 50°C, the ETHSP cells still had a survival rate of about 10%, almost 1000-fold higher as compared with the ET32A cells, which had only 0.01% survival rate. Heating at 50°C for 1.5 h dropped the survival rate of the ETHSP cells to 1.8%, whereas no ET32A cells survived under the same condition. It was evident that 0.02% ETHSP cells could survive 2 h exposure to 50°C (Figure 4). It appeared that the transformed E. coli cells (ETHSP) producing the
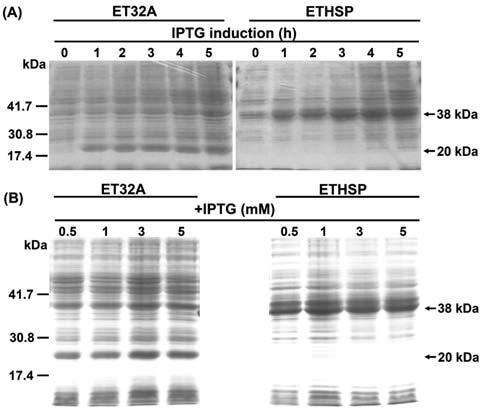
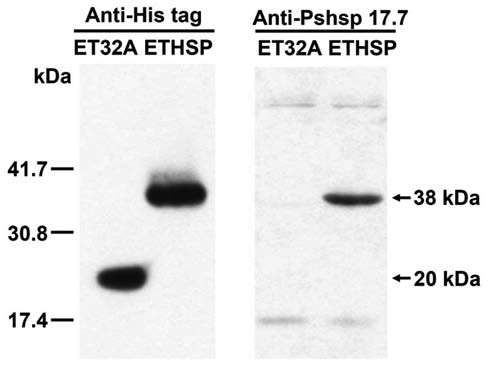
Figure 1. SDS-PAGE analysis of total proteins extracted from ET32A cells containing the pET32a expression vector and the ETHSP cells with the recombinant plasmid pETHSP overex-pressing recombinant Oshsp18.0-CII. A: Total proteins extracted from ET32A and ETHSP cells after 0, 1, 2, 3, 4, and 5 hour (h) 1 mM IPTG induction; B: Total proteins extracted from ET32A and ETHSP cells after 0.5, 1, 3, and 5 mM IPTG induction for 4 h. Ten [iL of protein samples were subjected to SDS-PAGE analysis. The recombinant protein expressed in ET32A and ETHSP cells was ~20 kDa and ~38 kDa in size, respectively.
by 1 mM IPTG induction (Figure 1B). The western blotting analysis showed that the IPTG induced Oshsp18.0-CII fusion protein in the transformed ETHSP cells was histidine-tagged and recognized by the polyclonal antibodies against Pshsp17.7, the 17.7 kDa class II sHSP of pea (Figure 2).
Figure 2. Immunological gel blots of the recombinant proteins produced in ET32A and ETHSP cells. Monoclonal antibodies against histidine tag (Anti-His tag) and polyclonal antibodies against the 17.7 kDa class II HSP of pea (Anti-Pshsp 17.7) were used for blotting. The His-tagged recombinant Trx TagTM thi-oredoxin protein of ~20 kDa in ET32A and ETHSP cells and the His-tagged Oshsp18.0-CII fusion protein of ~38 kDa in ETHSP cells were detected after incubation 4 h with 1 mM IPTG.
Growth rates of the transformed E. coli cells
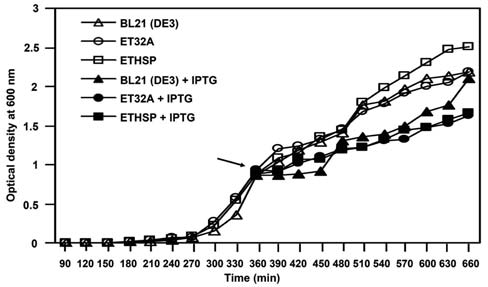
The ET32A and ETHSP cells had similar growth rates at 37°C when compared with the non-transformed E. coli BL21 (DE3) cells (Figure 3, open symbols), although slightly higher OD600 values were obtained in ETHSP cells after growth at 37°C for 480 min. After 1 mM IPTG in-duction, similar growth rates of the non-transformed BL21 (DE3), ET32A, and ETHSP cells were found (Figure 3, closed symbols). The growth rates of the above three cul-ture cells, however, were lower when subjected to 1 mM IPTG treatment than without IPTG. Overall, we observed no difference in the growth of these three E. coli cells at 37°C.
Thermotolerance of the E. coli cells containing the Oshsp18.0-CII fusion protein
Figure 3. Growth of wild-type and transformed E. coli BL21 (DE3) cells at 37°C with or without IPTG. Cultures of E. coli BL21 (DE3) cells (A and ▲), ET32A cells (O and •) carrying thepET32a vector, and ETHSP cells (口 and ■) carrying the recombinant pETHSP plasmid were grown at 37°C for 10 h. When OD600 reached to 0.8-1.0 (6 h after growth), half of the cultures were added with (closed symbols) or without (open symbols) IPTG to a final concentration of 1 mM, and subjected to further growth at 37°C for 4 h. Arrow indicates the addition of IPTG. Optical density at 600 nm was measured for cell growth.
To investigate the molecular chaperone function of Oshsp18.0-CII in vivo, the attenuation in lethality at 50°C was checked for the E. coli cells producing the re-combinant Oshsp18.0-CII protein. As shown in Figure 4, after exposing cells to 50°C for 0.5 h, the survival rate of ET32A cells dropped to 0.4%, whereas that of the ETHSP