|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies (2012) 53: 401-414.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sinosenecio jiangxiensis (Asteraceae), a new species from Jiangxi, China
|
|
|
|
|
|
|
|
Ying LIU1* and Qin-Er YANG2
|
|
|
|
|
|
|
|
1School of Life Sciences, Sun Yat-sen University, Xingangxi Road, Haizhu District, Guangzhou 510275, People's Republic of China
2Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese
Academy of Sciences, Xingke Road, Tianhe District, Guangzhou 510650, People's Republic of China
|
|
|
|
|
|
|
|
(Received January 26, 2012; Accepted March 30, 2012)
|
|
|
|
|
|
|
|
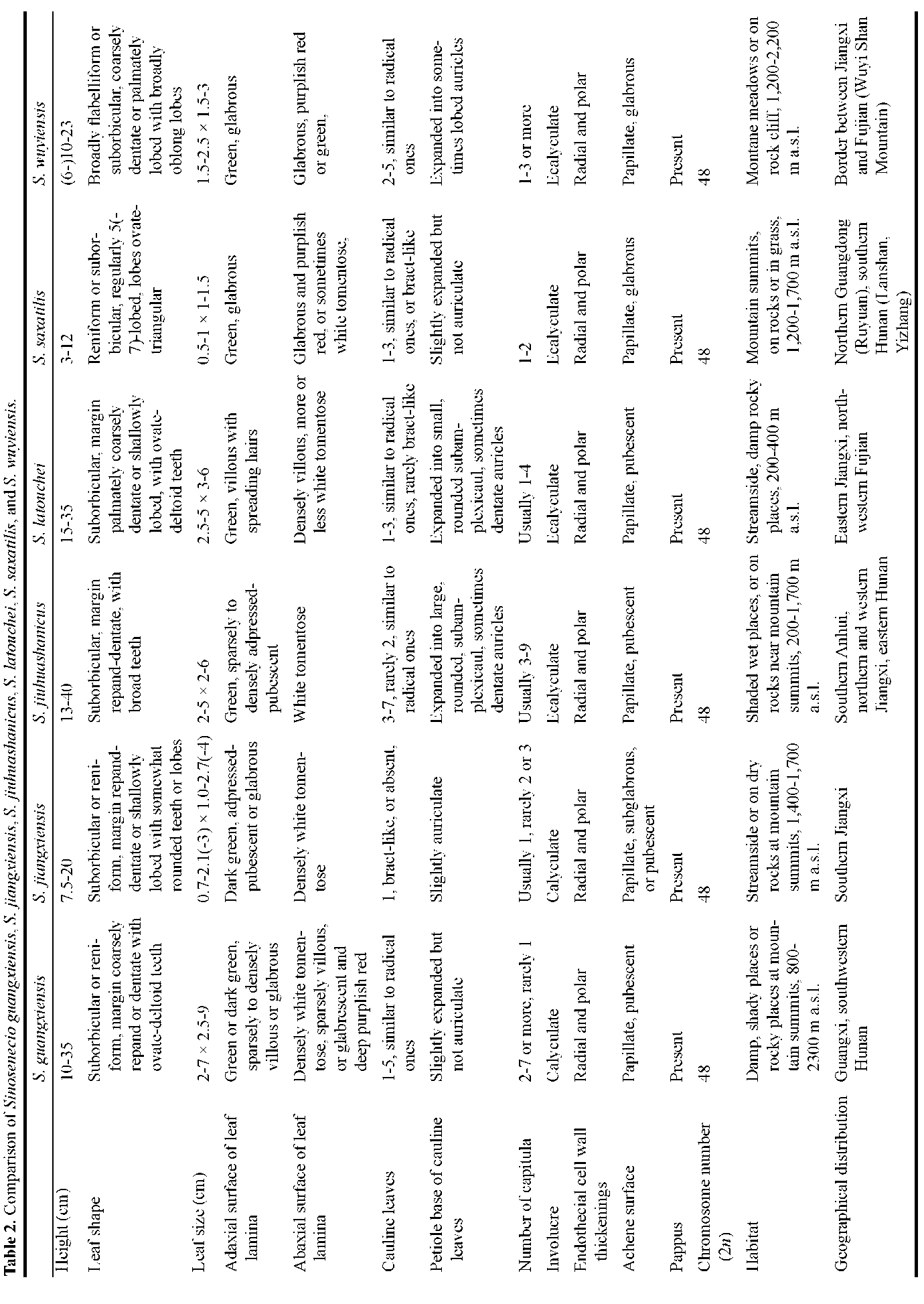
ABSTRACT. Sinosenecio jiangxiensis Y. Liu & Q. E. Yang, a new species from Jiangxi, China, is described and illustrated and its chromosome number (2n = 48) is reported. The karyotype is formulated as 2n =42m + 4sm + 2st (2sat). Sinosenecio jiangxiensis is similar to S. guangxiensis C. Jeffrey & Y. L. Chen and S. latouchei (J. F. Jeffrey) B. Nord. in leaf shape, abaxial leaf surface indumentum and achene pubescence, but differs in its smaller stature, smaller basal leaves, stem leafless or bearing only 1 bract-like leaf near the middle and thus more typically scapiform, and usually solitary capitula. Analysis of ITS sequences further confirmed the distinction of S. jiangxiensis and also revealed that S. jiangxiensis is closely related to S. guangxiensis, S. jiuhuashanicus C. Jeffrey & Y L. Chen, S. latouchei, S. saxatilis Y. L. Chen, and S. wuyiensis Y L. Chen, with S. jiuhuashanicus, S. latouchei and S. muyiemis being the closest relatives. A detailed comparison of these species is made, and a key to them is provided.
|
|
|
|
|
|
|
|
Keywords: Asteraceae; Chromosome number; Karyotype; Senecioneae; Sinosenecio jiangxiensis.
|
|
|
|
|
|
|
|
|
(2011), in which Sinosenecio is circumscribed as including species with x = 30 as well as those with x = 24 (rarely 13), but not including Nemosenecio.
|
|
|
|
Sinosenecio B. Nord. (Senecioneae-Asteraceae) as most recently circumscribed includes 41 species, all occurring in China, mainly in the central and southwestern regions, with only two extending into Myanmar, Thailand, and Vietnam (Chen et al., 2011). Several lines of evidence from cytology (Liu and Yang, 2011a), floral micromor-phology (Liu and Yang, 2011b), and molecular systemat-ics (Wang et al., 2009; Liu, 2010) strongly indicate that Sinosenecio is not a monophyletic group and needs further re-circumscription. It appears that only those species of Sinosenecio with a base chromosome number of x = 30 and with strictly polar endothecial cell wall thickenings, which include the type species of the genus, S. homogyni-phyllus (Cumm.) B. Nord., should be retained within Sino-senecio as redefined; species with x = 24 (rarely 13) and with polar and radial endothecial cell wall thickenings may represent an undescribed genus or should be transferred to Nemosenecio (Kitam.) B. Nord. (also with x = 24) (Liu, 2010; Liu and Yang, 2011a, b). As the relationships between the second group of Sinosenecio and Nemosenecio have as yet not been satisfactorily resolved, no formal taxonomic treatment at the generic level has been made (Nordenstam and Pelser, 2011). In this study we therefore follow the generic concept and delimitation of Chen et al.
|
|
|
|
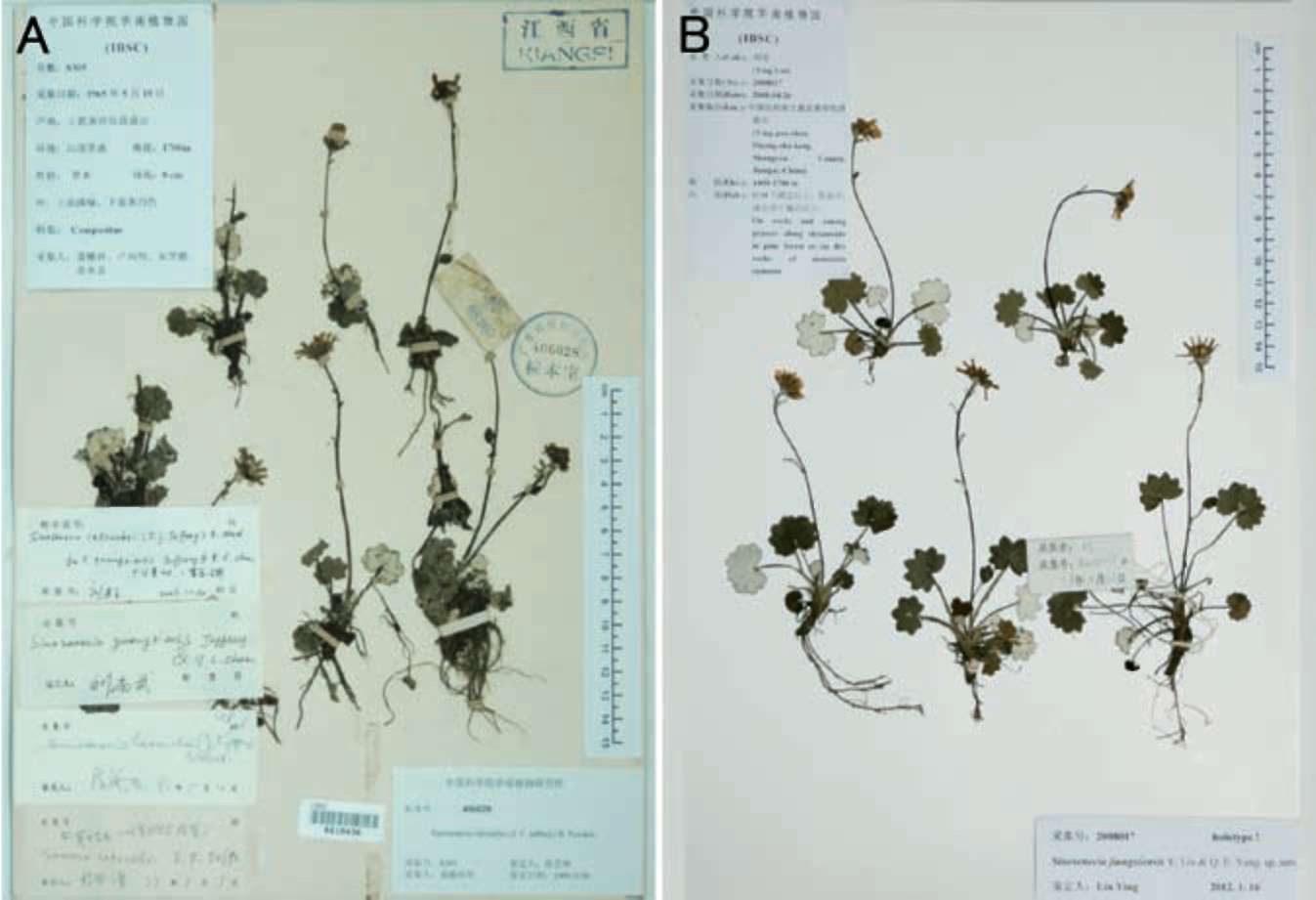
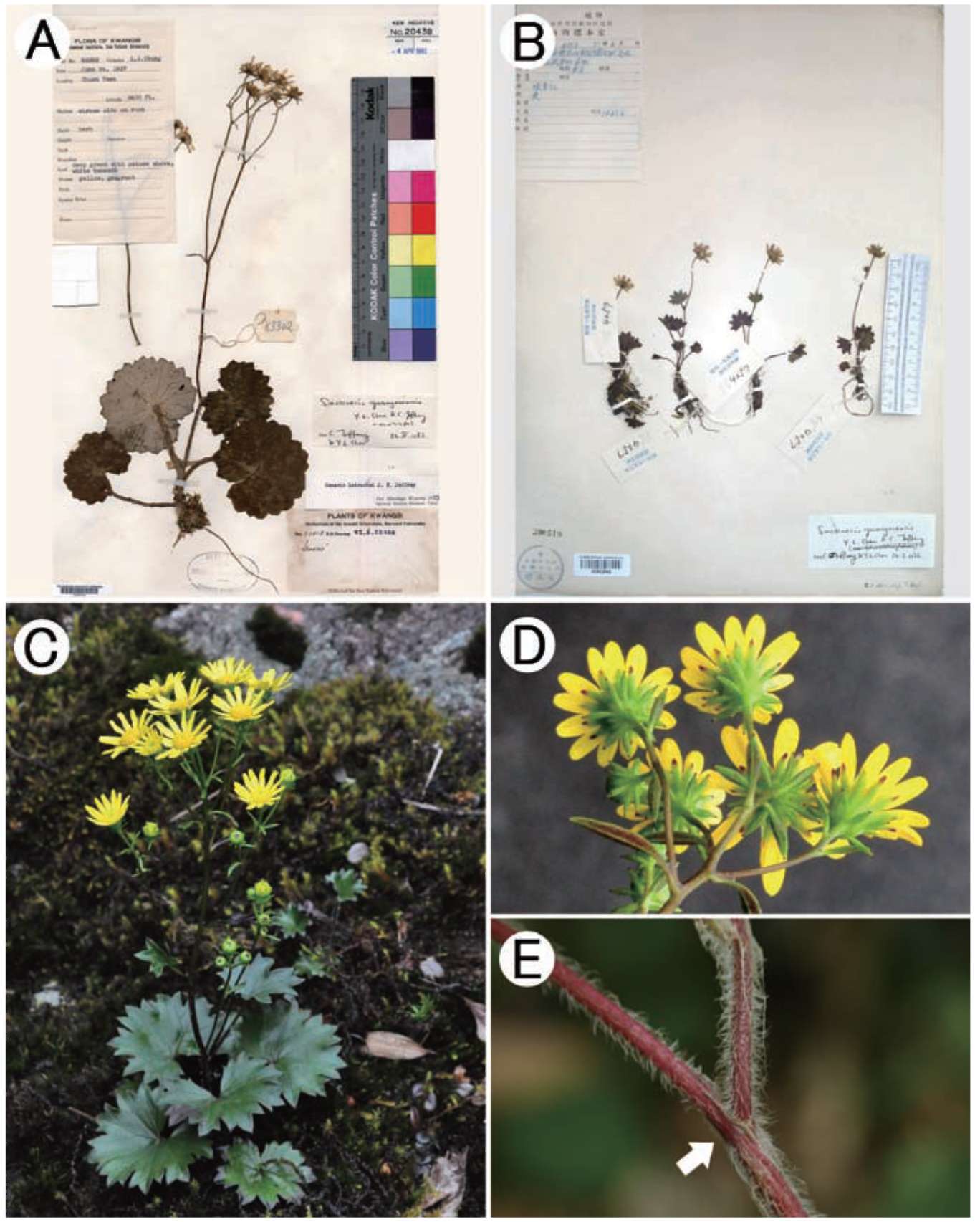
When checking specimens of Sinosenecio deposited in the major Chinese herbaria, a collection, Min-xiang Nie et al. 8305 (IBSC, KUN, PE) (Figure 1A, only one sheet is shown) from Shangyou County, Jiangxi Province, China, caught our attention. This collection had been variously identified as S. guangxiensis C. Jeffrey & Y L. Chen, S. latouchei (J. F. Jeffrey) B. Nord., and S. jiuahuashanicus C. Jeffrey & Y. L. Chen. This collection was also sampled as S. guangxiensis by Wang et al. (2009) for a molecular phylogenetic analysis of the Nemosenecio-Sinosenecio-Tephroseris assemblage using sequence data of the ITS region of the nuclear ribosomal DNA (the authors wrongly attributed the provenance of this collection to Guangxi). Our herbarium and field observations, however, revealed that the plant in question does not match well with any of the above three species, leading us to recognize it as a new species. We also revisited the phylogeny of the genus Sinosenecio as reconstructed by Wang et al. (2009), and included in our analysis more putative relatives of the new species to further unravel its phylogenetic relationships.
|
|
|
|
Sinosenecio jiangxiensis Y Liu & Q. E. Yang, sp. nov.― TYPE: CHINA. Jiangxi, Shangyou County, Huang-sha-keng, Ying-pan-shan, alt. 1,400-1,700 m, on rocks and in grass along streamside in pine forests or on dry rocks
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
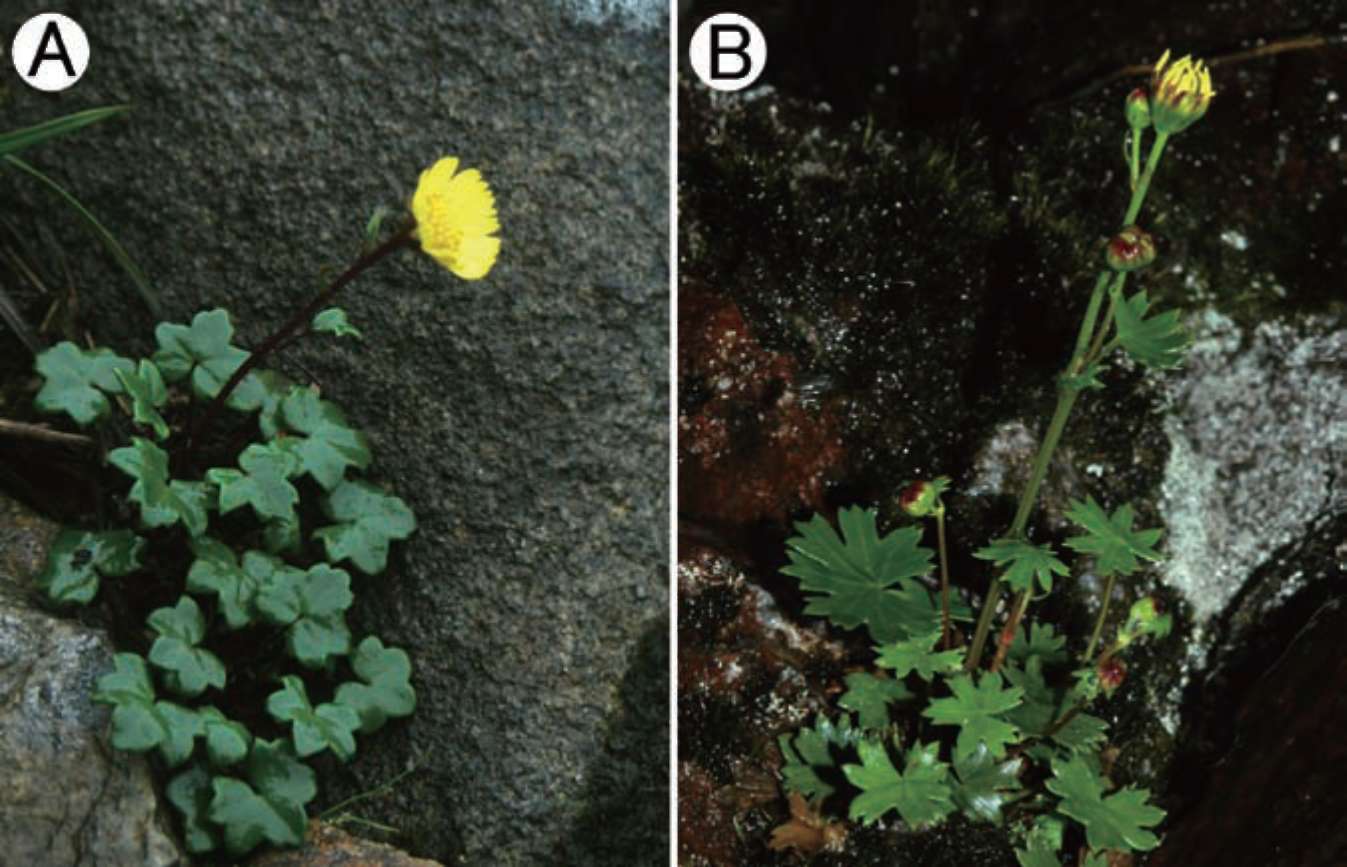
Figure 1. Sinosenecio jiangxiensis. A, China, Jiangxi, Shangyou, Huang- sha-keng, Ying-pan-shan, Min-xiang Nie et al. 8305 (paratype, IBSC); B, Same locality, Ying Liu 2008017 (holotype, IBSC).
|
|
|
|
|
|
|
|
|
at mountain summits, 26 Apr 2008, Ying Liu 2008017 (holotype, IBSC; isotypes, HAST, SYS).江西蒲兒根
Figures 1, 2, 3
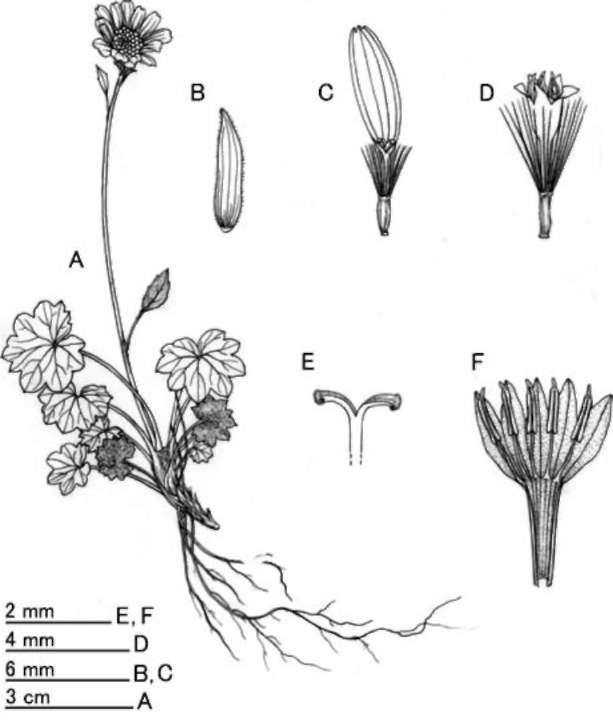
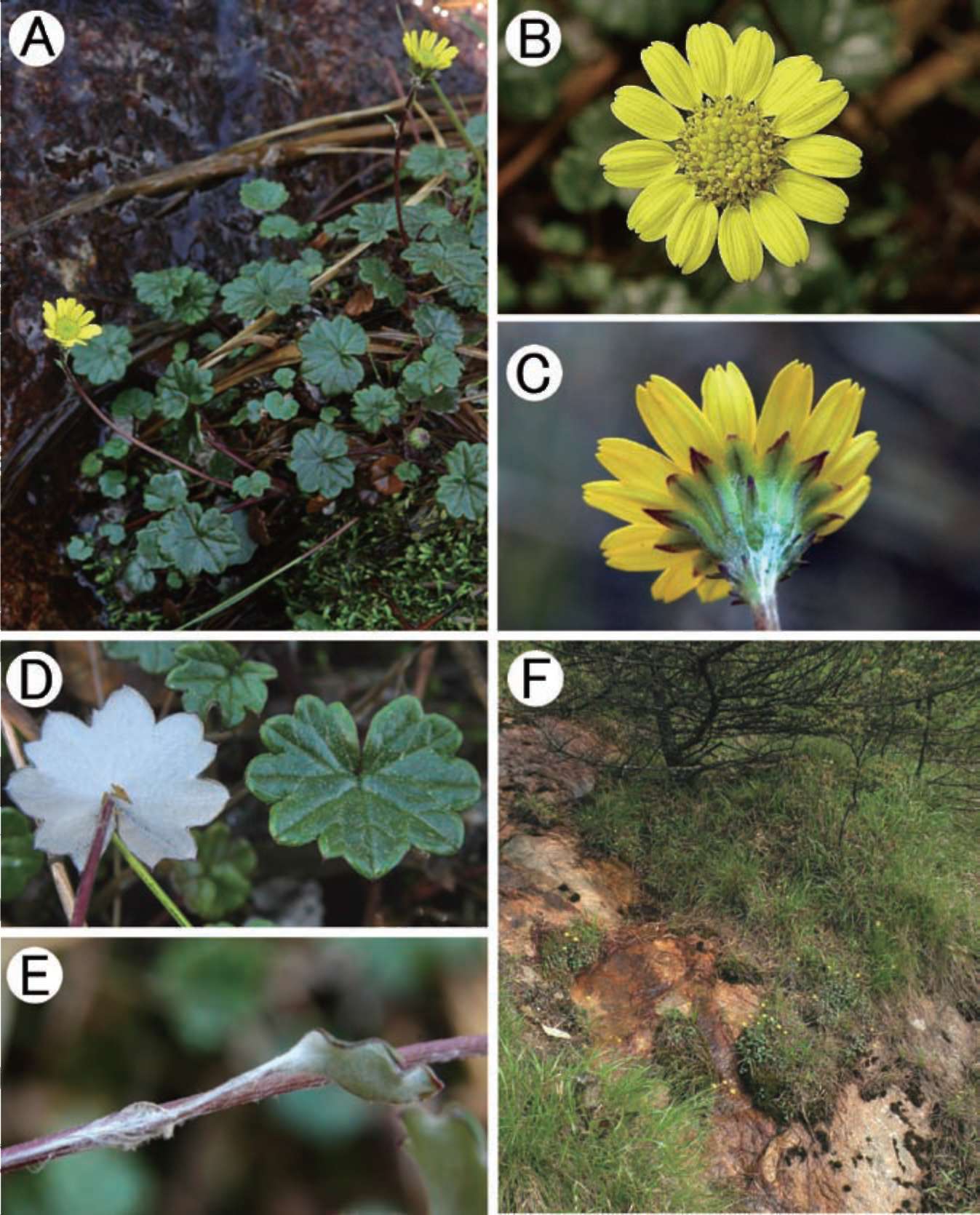
Description: Scapigerous herbs. Rhizomes 3-5 mm in diam., collar lanate-tomentose, clad in persistent petiole bases. Stems solitary, rarely several, scapiform, 7.5-20 cm tall, simple, sparsely white tomentose or sometimes glabrescent. Radical leaves 5-10, rosulate, petiolate; petiole 1-7 cm long, sparsely white tomentose, glabrescent, basally expanded; lamina suborbicular or reniform 0.7-2.1(-3) x 1.0-2.7(-4) cm, thick papery, abaxially densely white floccose-tomentose, adaxially dark green, appressed pubescent and sparsely to densely white tomentose or sometimes glabrescent, palmately 5-7-veined, base cordate to deeply cordate, margin repand-dentate or shallowly lobed with broad, somewhat rounded mucronulate teeth or lobes. Cauline leaf absent or 1 in middle or lower part of stem, bract-like, short-petiolate; petiole basally expanded and slightly auriculate; lamina ovate to lanceolate, abaxially densely white tomentose, margin entire or 3-5-dentate. Capitula solitary, rarely 2 or 3, ca. 2 cm across the rays. Peduncle bracteate; bracts 1-3, ovate-lanceolate, entire or 2- or 3-dentate, usually sessile distally. Involucre campanulate, ca. 9 x 7 mm, calyculate with 3-7 bracteoles; bracteoles linear, 3-5 mm long, abaxially densely white tomentose. Phyllaries ca. 13, oblong-lanceolate, 6-7 x 1.5-
|
|
|
|
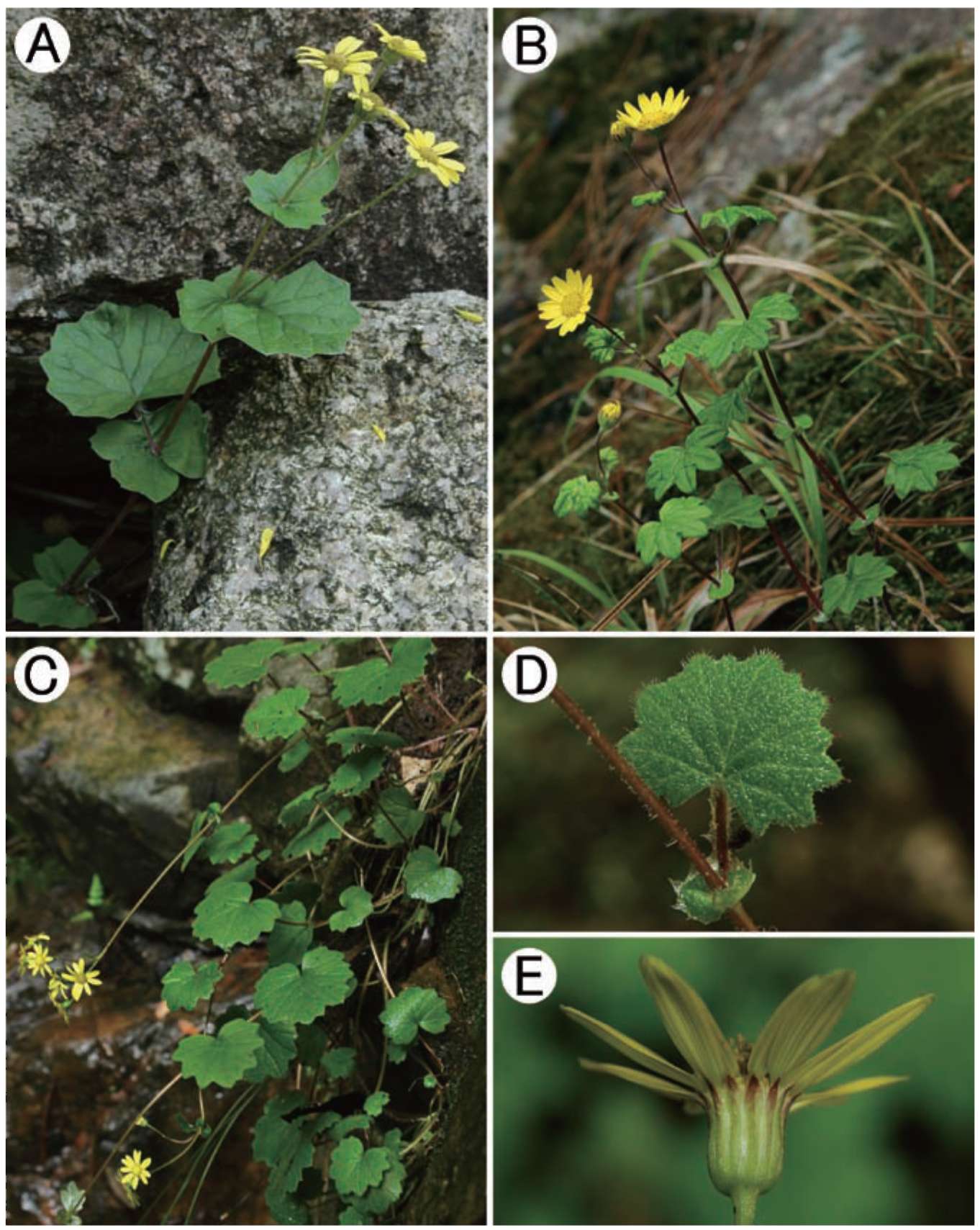
Figure 2. Sinosenecio jiangxiensis Y. Liu & Q. E. Yang. A, Habit; B, Phyllary; C, Ray floret; D, Disc floret; E, Style-arms; F, Dissected corolla of a disc floret showing stamens (All from Ying Liu 2008017, IBSC).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIU and YANG ― Sinosenecio jiangxiensis, a new species from China
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 3. Sinosenecio jiangxiensis. A, Habit; B, Capitulum (top view); C, Capitulum (bottom view showing phyllaries and bracteoles); D, Lamina of radical leaf (right: adaxial side; left: abaxial surface); E, Cauline leaf showing auricle; F, Habitat (All from the type locality and vouched by Ying Liu 2008017, HAST, IBSC, SYS).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
2.5 mm, herbaceous, sparsely white tomentose, margin broadly scarious, apically acute or acuminate, reddish purple, ciliate. Ray florets ca. 13; corolla tube 2-2.5 mm long, glabrous; rays yellow, oblong, 6-7 x 2-3 mm, 4-7-veined, apically 3-denticulate, obtuse. Disc florets many; corolla yellow, ca. 4 mm long; tube 1.5-1.7 mm long; limb 1.5-2 mm long; lobes lanceolate, 1 mm long, apically acute. Anthers ca. 1 mm long, basally obtuse, appendages ovate-triangular. Style branches ca. 0.5 mm long. Achenes 1.5-1.7 mm long, pubescent, rarely subglabrous, papillate. Pappus white, ca. 3 mm long.
|
|
|
|
|
Additional specimens examined. CHINA. Jiangxi, Shangyou County, Huang-sha-keng, Ying-pan-shan, alt. 1,700 m, on rocks and in grass at mountain summits, 19 May 1965, Min-xiang Nie et al. 8305 (IBSC, KUN, PE).
|
|
|
|
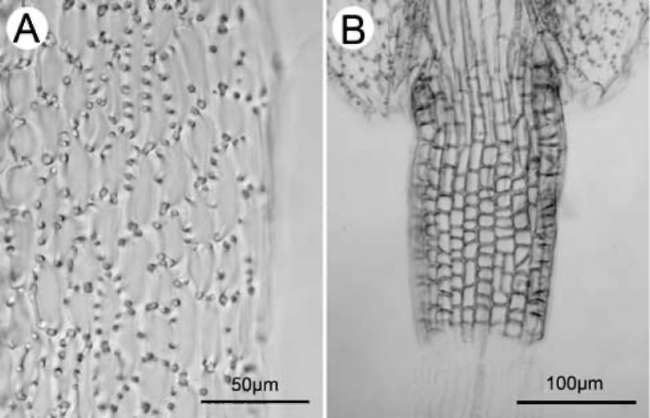
Figure 5. Endothecial cell wall thickenings (A) and filament collar (B) of Sinosenecio jiangxiensis. A, Radial and polar thickenings; B, Uniformly sized cells of filament collar (All from Ying Liu 2008017, IBSC).
|
|
|
|
Etymology. The specific epithet 'jiangxiensis, is derived from Jiangxi Province in southeast China, where the type specimen was collected.
|
|
|
|
|
|
|
|
Phenology. Flowering from late April to May; fruiting May to June.
|
with x = 24, including the putative relatives (S. guangx-iensis, S. jiuhuashanicus, and S. latouchei) (Liu and Yang, 2011b). In species of Sinosenecio with x = 30, the thickenings are strictly polar (Liu and Yang, 2011b). Based on the type of endothecial cell wall thickenings, S. jiangxiensis was expected to have a base chromosome number of x =24 (see below). The filament collar of S. jiangxiensis consisted of uniformly sized cells (Figure 5B), a character which was previously considered to be one of the diagnostic features of Sinosenecio (Nordenstam, 1978; Jeffrey and Chen, 1984), but now has been revealed to be a fairly generalized character in the subtribe Tussilagininae sensu lato (Liu, 1999; Nordenstam et al., 2009; Liu and Yang, 2011b).
|
|
|
|
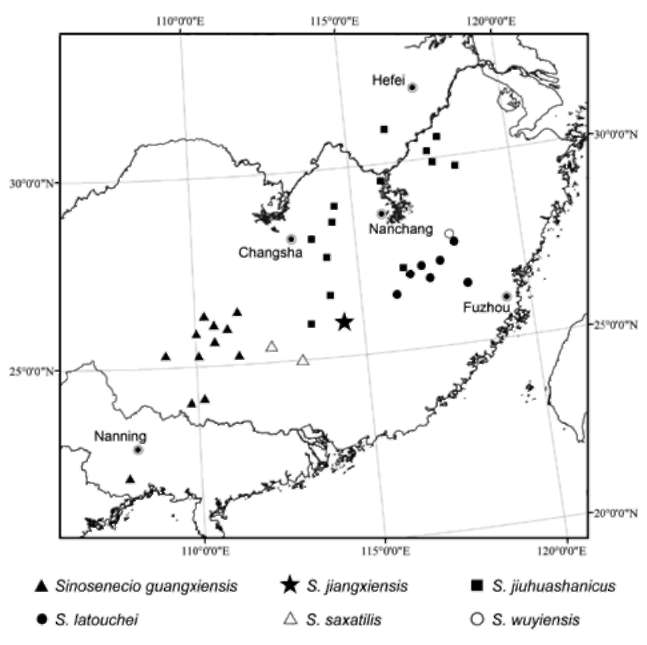
Distribution and habitat. Sinosenecio jiangxiensis is known only from the type locality (Figure 4), growing on rocks or in grass along streamside in pine forests, or on dry rocks at mountain summits at altitudes between 1,4001,700 m above the sea level.
|
|
|
|
Floral micromorphological characters. To observe the anther endothecial cell wall thickenings and the filament collar of Sinosenecio jiangxiensis, we followed the methods described in Liu and Yang (2011c).
|
|
|
|
One population [Ying Liu 2008017 (HAST, IBSC, SYS)] was examined. The endothecial cell wall thickenings were both radial and polar (Figure 5A). This type of thickenings also occurs in other species of Sinosenecio
|
|
|
|
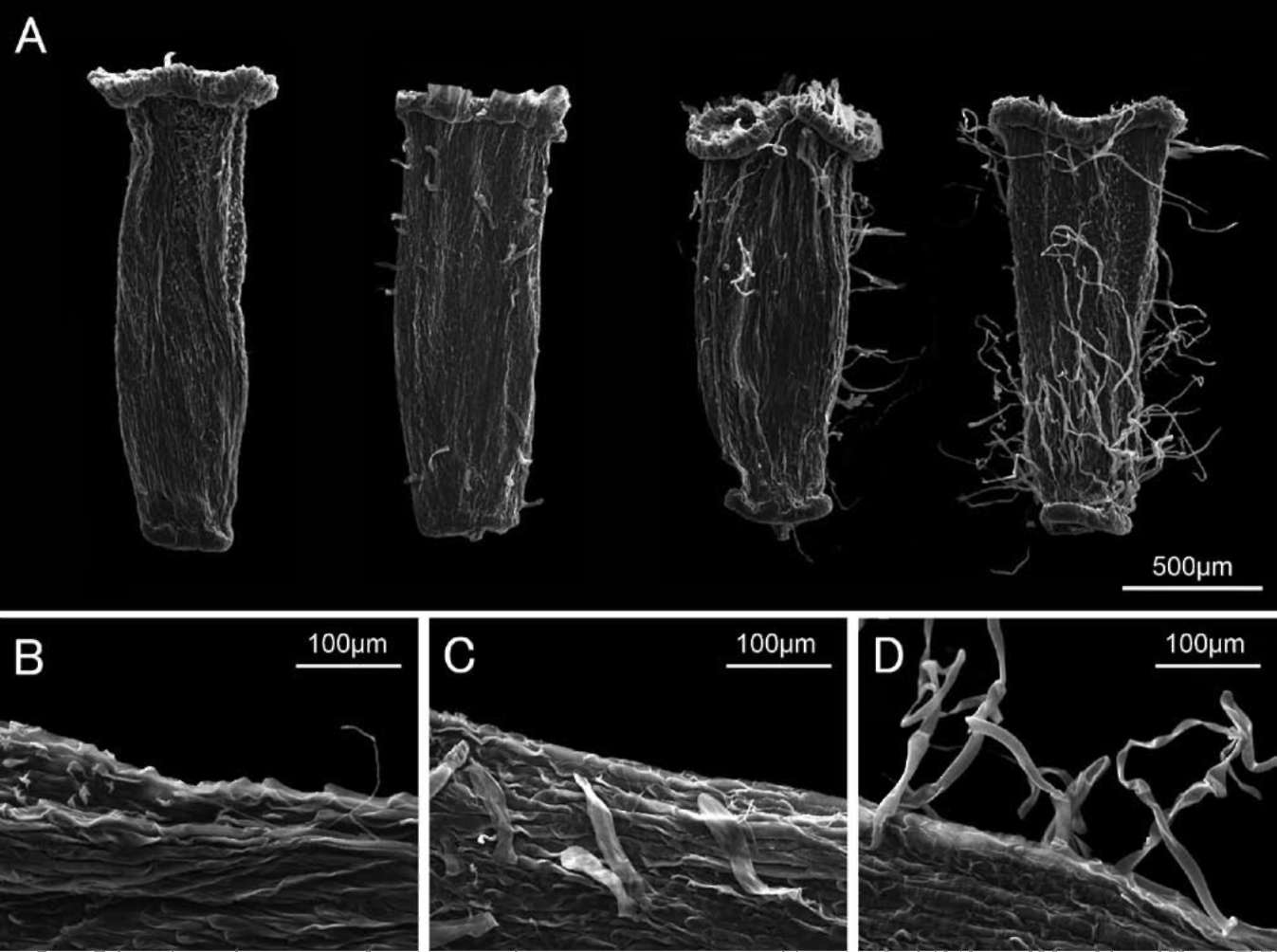
Achene surface features. To determine the potential taxonomic applicability of achene surface features in Sino-senecio jiangxiensis and its putative close relatives, we examined the achenes of several populations [including S. guangxiensis (Ying Liu 2008021, IBSC), S. jiangxien-sis (Ying Liu 2008017, IBSC), S. jiuhuashanicus (Ying Liu & Tao Deng 2008018, Ying Liu 2008020, IBSC), and S. latouchei (Ying Liu & Tao Deng 2008010, 2008013, IBSC)] using a scanning electron microscope (SEM). For each population, ten capitula from each of different individuals, and five to eight achenes from each capitulum, were first observed using light microscopy. The achenes representing the range of variation within a population were then mounted directly on stubs, gold-coated and photographed under a JSM-6360LV SEM.
|
|
|
|
|
|
|
|
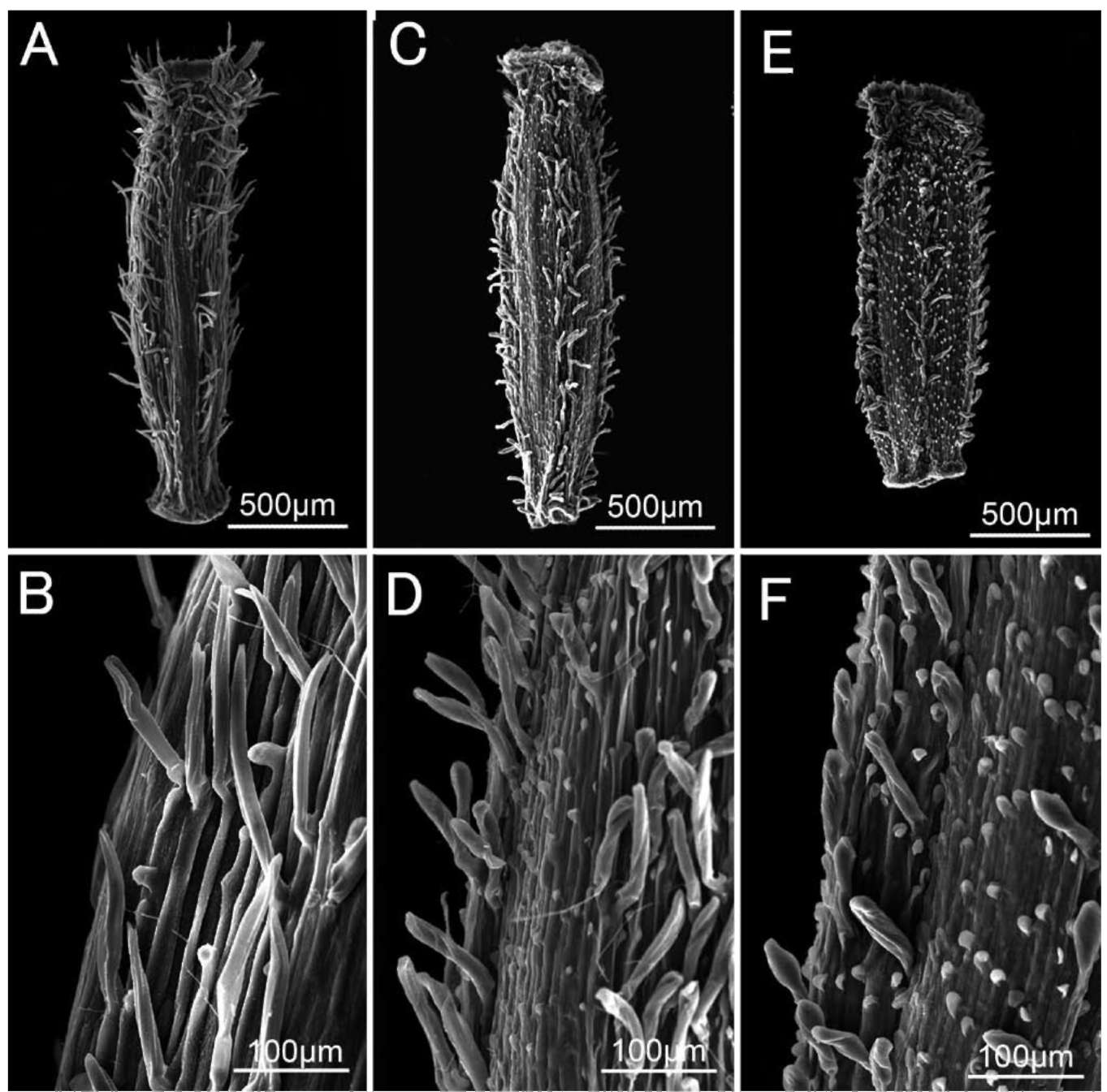
Achenes of all four species examined were papillate and more or less pubescent on the surface (Figures 6-7, not all the results are shown), although pubescence of the achenes in S. jiangxiensis showed some variation within a population, sometimes even in the same capitulum (see below). The achenes of the other species were all reported to be smooth and glabrous with only a few exceptions: papillate but glabrous in Sinosenecio fangianus Y. L. Chen, S. saxatilis Y. L. Chen, S. sungpanensis (Hand.-Mazz.) B.
|
|
|
|
Figure 4. Distribution of Sinosenecio jiangxiensis and related species.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIU and YANG ― Sinosenecio jiangxiensis, a new species from China
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 6. Achene surface features of Sinosenecio jiangxiensis. A, Achenes, showing variation of the pubescence; B, Achene papillate and subglabrous; C, Achene papillate and pubescent with twin hairs; D, Achene papillate and pubescent with long, multicellular hairs
(All from Ying Liu 2008017, IBSC).
|
|
|
|
|
|
|
|
Nord. and S. wuyiensis Y. L. Chen; and papillate and pubescent only in the ray florets and smooth and glabrous in the disc florets in S. oldhamianus (Maxim.) B. Nord. (Liu, 2010). The common achene surface features shared by S. jiangxiensis with its relatives, as revealed here, therefore lend support to their close relationships. The achene pubescence of S. jiangxiensis was slightly different from that of its putative relatives, even varying within the same capitulum from being subglabrous, pubescent with twin hairs, to pubescent with long, multicellular hairs (Figure 6). The achenes of S. guangxiensis, S. jiuhuashanicus and S. latouchei were pubescent with only twin hairs (Figure 7B, D, and F), a character seemingly quite stable within and between populations.
|
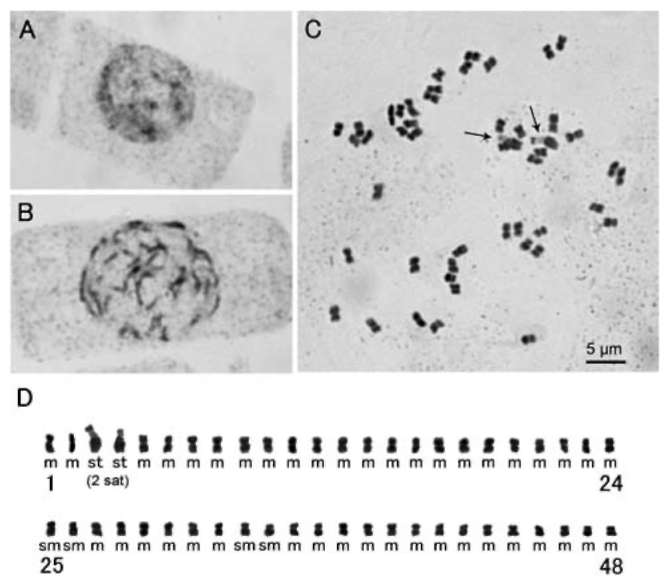
to be 2n = 48 (Figure 8C), as predicted on the basis of the type of endothecial cell wall thickenings of the species. The chromosomes ranged in length from 2.59 to 1.16 [im, and the total karyotype length was 83.35 [im. According to the nomenclature of chromosomes of Levan et al. (1964), S. jiangxiensis had 42 median-centromeric (m), 4 sub-median-centromeric (sm), and 2 subterminal-centromeric (st) satellited chromosomes (Figure 8D), i.e. 2n = 48 = 42m + 4sm + 2st (2sat). In species of Sinosenecio with x = 24, S. guangxiensis, S. jiangxiensis, S. jiuhuashanicus, S. latouchei, S. saxatilis and S. wuyiensis have the same chromosome number as well as remarkably similar chromosome morphology and size; their chromosomes are considerably smaller than those in most of the remaining species with x = 24 (Liu and Yang, 2011a; this study). Cy-tologically, these species are closely related.
|
|
|
|
Cytology. One population (Ying Liu 2008017) of Sino-senecio jiangxiensis was cytologically studied. Carbol fuchsin root tip squashes were made following Liu and Yang (2011c). In the interphase nucleus, a few darkly stained condensed bodies were observed, but their boundaries were not clear (Figure 8A). The prophase chromosomes presented a nearly continuous condensation pattern (Figure 8B). The metaphase chromosomes were counted
|
|
|
|
Molecular phylogenetic analyses. One collection of Sinosenecio jiangxiensis (as S. guangxiensis), Min-xiang Nie et al. 8305, was used by Wang et al. (2009) in a phylogenetic analysis of the Nemosenecio-Sinosenecio-Tephroseris assemblage. Their results showed that some species of Sinosenecio [all revealed to have x = 24, rarely
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 7. Achene surface features of Sinosenecio guangxiensis (A-B), S. jiuhuashanicus (C-D) and S. latouchei (E-F). A-F, Achenes
papillate and pubescent with twin hairs (A and B from Ying Liu 2008021, IBSC; C and D from Ying Liu 2008020, IBSC, PE; E and F from Ying Liu & Tao Deng 2008013, IBSC, PE).
|
|
|
|
|
|
|
|
x = 13 by Liu and Yang (2011a)] and three species of Nem-osenecio were nested in a clade sister to Tephroseris (Wang et al., 2009). Within this clade, the specimen Min-xiang Nie et al. 8305 and two accessions of true S. guangxiensis formed a strongly supported subclade sister to the remaining species. The results of Wang et al. (2009) therefore indicate a close relationship between S. jiangxiensis and S. guangxiensis. Regrettably other putative close relatives of S. jiangxiensis were not sampled in their analysis. To further unravel the systematic affinities of S. jiangxiensis, we included in our ITS analysis those species of Sinosenecio
|
shown to be close relatives of S. jiangxiensis on the basis of morphological and chromosomal characters. Different populations spanning the range of morphological variation were sampled as much as possible.
|
|
|
|
ITS sequences of nine accessions representing six species included in our analysis were newly obtained; the remainder sequences were downloaded from GenBank. Tephroseris kirilowii (Turcz. ex DC.) Holub was used as outgroup; 13 species of Sinosenecio (19 populations) with x = 24 and two species of Nemosenecio were used as ingroups. The selection of species was based on our
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIU and YANG ― Sinosenecio jiangxiensis, a new species from China
|
|
|
|
|
|
|
|
|
|
recent systematic studies of Sinosenecio (Liu, 2010; Liu and Yang, 2011a, b). Voucher information and GenBank accession numbers are given in Table 1. Total DNA extraction followed Doyle and Doyle (1987). The primers ITS1 and ITS4 (White et al., 1990) were used to amplify and sequence the ITS region. Polymerase chain reaction and sequencing reactions were performed in Eppendorf Mastercycler gradient (Hamburg, Germany) following the procedure described in Wang et al. (2009) or with modifi-cations thereof. All the sequences were first aligned with Bioedit version 7.0.9 (Hall, 1999) and then adjusted manu-ally. Bayesian inference (BI), maximum likelihood (ML) and maximum parsimony (MP) methods for phylogenetic estimation were performed using MrBayes 3.1.2 (Huelsen-beck et al., 2001), PhyML version 3.0 (Guindon and Gas-cuel, 2003) and PAUP* version 4.0b10 (Swofford, 2003), respectively. The evolutionary model was selected using Modeltest version 3.7 (Posada and Crandall, 1998).
|
|
|
|
|
|
|
|
Figure 8. Interphase nucleus (A), mitotic prophase (B), meta-phase (C, 2n = 48) chromosomes, and karyotype (D) of Sino-senecio jiangxiensis (All from Ying Liu 2008017, HAST, IBSC, SYS). Arrows indicate satellited chromosomes.
|
The aligned matrix contained 541 characters, of which 400 were constant and 61 were parsimony-informative. The best-fit model selected for this data set was GTR + G using the Akaike Information Criterion (AIC). Trees gen-
|
|
|
|
|
|
|
|
Table 1. Sources of plant material analyzed and accession numbers in GenBank. Generic circumscription and species treatment followed Chen et al. (2011).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sinosenecio confervifer (H. Lev.) Y. Liu & Q. E. Yang
|
|
Zheng-yu Liu 001 , Nanchuan, Chongqing
|
|
|
|
|
S. changii (B. Nord.) B. Nord. & Pelser
|
|
|
|
|
|
|
S. euosmus (Hand.-Mazz.) B. Nord.
|
|
Liu 20040620-2, Emei, Sichuan
|
|
|
|
|
S. fanjingshanicus C. Jeffrey & Y L. Chen
|
|
WLK 422, Songtao, Guizhou
|
|
|
|
|
S. globiger (Chang) B. Nord.
|
|
Liu 818, Nanchuan, Chongqing
|
|
|
|
|
S. guangxiensis C. Jeffrey & Y. L. Chen
|
|
Gao-05001, Longsheng, Guangxi
|
|
|
|
|
|
|
Tian-gang Gao sino01, Longsheng, Guangxi
|
|
|
|
|
|
|
Ying Liu 2008021 (IBSC), Xingan, Guangxi
|
|
|
|
|
S. jiangxiensis Y. Liu & Q. E. Yang
|
|
Min-xiang Nie et al. 8305, Shangyou, Jiangxi [sampled as S. guangxiensis in Wang et al. (2009)]
|
|
|
|
|
|
|
Ying Liu 2008017 (HAST, IBSC), Shangyou, Jiangxi
|
|
|
|
|
S. jiuhuashanicus C. Jeffrey & Y. L. Chen
|
|
Ying Liu & Tao Deng 2008018 (IBSC, PE), Pingxiang, Jiangxi
|
|
|
|
|
|
|
Ying Liu & Tao Deng 2008019 (IBSC, PE), Liuyang, Hunan
|
|
|
|
|
|
|
Ying Liu 2008020 (IBSC, PE), Huang Shan, Anhui
|
|
|
|
|
S. latouchei (J. F. Jeffrey) B. Nord.
|
|
Ying Liu & Tao Deng 2008010 (IBSC, PE), Nanping, Fujian
|
|
|
|
|
|
|
Ying Liu & Tao Deng 2008013 (IBSC, PE), Wuyi, Fujian
|
|
|
|
|
S. oldhamianus (Maxim.) B. Nord.
|
|
|
|
|
|
|
|
|
Ying Liu 2008024 (IBSC, PE), Lanshan, Hunan
|
|
|
|
|
S. septilobus (Chang) B. Nord.
|
|
Liu 800, Nanchuan, Chongqing
|
|
|
|
|
|
|
Ying Liu & Tao Deng 2008014 (IBSC, PE), Wuyi, Fujian
|
|
|
|
|
Nemosenecio (Kitam.) B. Nord.
|
|
|
|
|
|
|
N. incisifolius (J. F. Jeffrey) B. Nord.
|
|
Liu 50, Jiangchuan, Yunnan
|
|
|
|
|
|
|
|
|
|
|
|
Tephroseris (Reichenb.) Reichenb.
|
|
|
|
|
|
|
T. kirilowii (Turcz. ex DC.) Holub
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
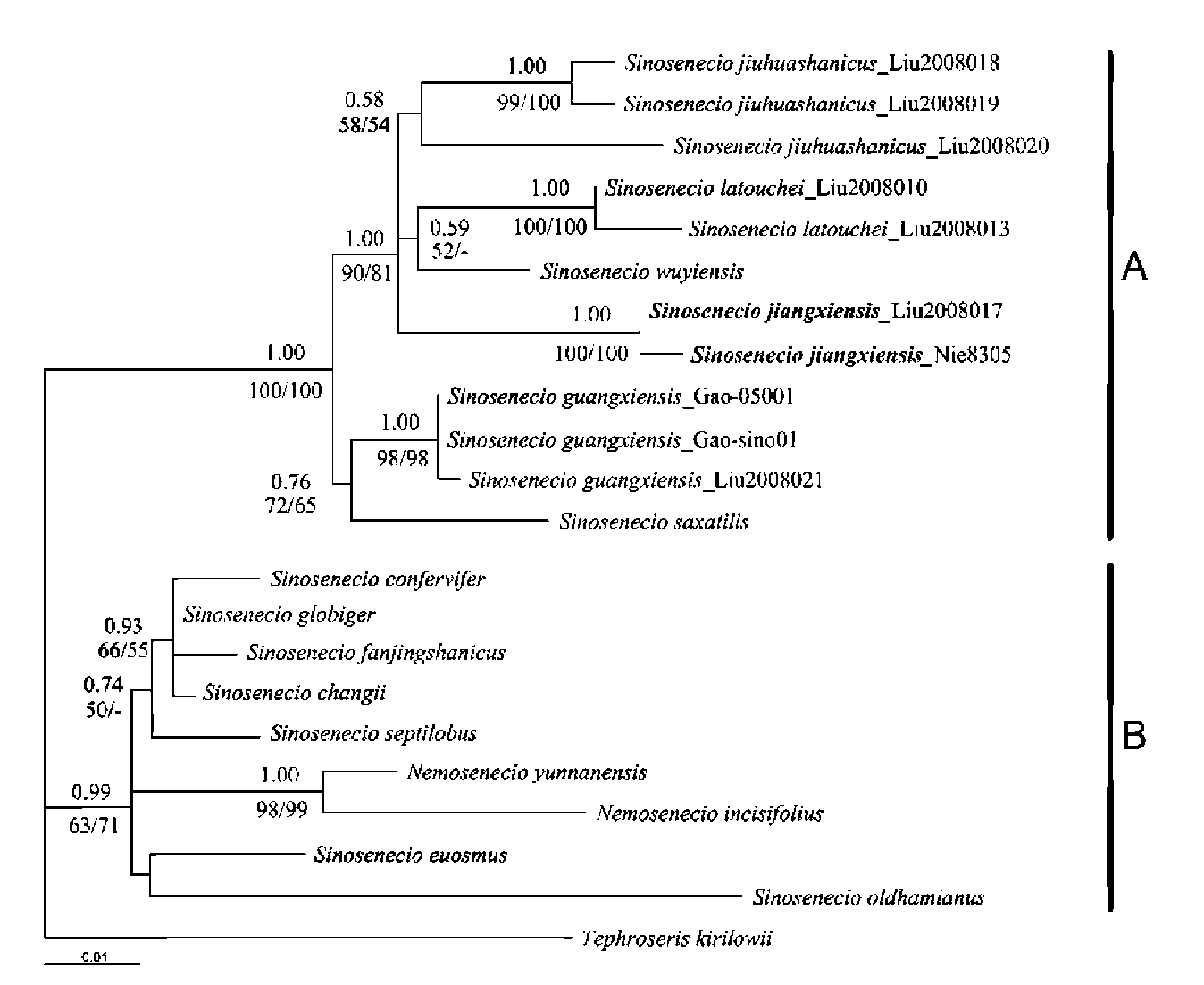
Figure 9. Phylogram of the maximum likelihood analysis of ITS data, showing the phylogenetic position of Sinosenecio jiangxiensis within the x = 24 species assemblage of the genus. Numbers above branches are Bayesian posterior probabilities and numbers below branches are bootstrap values obtained in the maximum likelihood (left) and maximum parsimony (right) analyses. Bootstrap values of < 50% are indicated by a A and B represent the two clades within the assemblage.
|
|
|
|
|
|
|
|
erated in Bayesian, maximum likelihood and maximum parsimony analyses of the ITS dataset had almost identical overall topologies. Only the ML tree is shown here (Figure 9). Two clades were resolved within the Sinosenecio-Ne-mosenecio assemblage. Sinosenecio jiangxiensis together with S. guangxiensis, S. jiuhuashanicus, S. latouchei, S. saxatilis and S. wuyiensis formed a strongly supported clade (clade A) [PP = 1.00, BS = 100% (ML) and 100% (MP)], whereas the remaining species of Sinosenecio were clustered with two species of Nemosenecio and formed a moderately supported clade (clade B) (PP = 0.99, BS = 63% and 71%) sister to clade A. Clade A consisted of two subclades. One, comprising S. jiangxiensis, S. jiuhuashani-cus, S. latouchei, and S. wuyiensis, received strong PP support (1.00) and moderate BS support (90% and 81%). The other, containing S. guangxiensis and S. saxatilis, was moderately to weakly supported (PP = 0.76, BS = 72%
|
and 65%). Three accessions of S. guangxiensis and two of S. latouchei formed well supported (PP = 1.00, BS > 98%) monophyletic lineages respectively, while the three accessions of S. jiuhuashanicus (a somewhat dimorphic species; see below) formed a weakly supported lineage (PP = 0.58, BS = 58% and 54%). The accession, Min-xiang Nie et al. 8305, sampled as S. guangxiensis by Wang et al. (2009), formed a monophyletic lineage with the here sampled accession of S. jiangxiensis, and rather independent of the S. jiuhuashanicus clade, the S. latouchei-S. wuyiensis clade, and the clade comprising S. guangxiensis and S. saxatilis.
|
|
|
|
The results of our ITS phylogenetic analysis, therefore, strongly support the recognition of S. jiangxiensis as a distinct species closely related to S. guangxiensis, S. jiuhuashanicus, S. latouchei, S. saxatilis and S. wuyiensis. The results also show S. jiangxiensis to be more closely related to S. jiuhuashanicus, S. latouchei and S. wuyiensis
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIU and YANG ― Sinosenecio jiangxiensis, a new species from China
|
|
|
|
|
|
|
|
|
than to S. guangxiensis and S. saxatilis, although morphologically S. guangxiensis appears to be one of the closest relatives of S. jiangxiensis.
|
wuyiensis (Figure 10B) differs from S. jiangxiensis in the leaf lamina abaxially glabrous (vs. abaxially densely white floccose-tomentose). Furthermore, involucres in S. saxa-tilis and S. wuyiensis are ecalyculate, and the achenes are glabrous, but in S. jiangxiensis the involucres are calycu-late, and the achenes are usually more or less pubescent.
|
|
|
|
Notes. Evidence from achene surface morphology, chromosome cytology, ITS phylogenetic analysis as well as geographical distribution shows that Sinosene-cio guangxiensis, S. jiangxiensis, S. jiuhuashanicus, S. latouchei, S. saxatilis and S. wuyiensis are closely related and constitute a natural group. Their achenes are papillate, their chromosomes are obviously smaller than those in most species of Sinosenecio with x = 24, and they are nested within a strongly supported clade on the ITS phylo-genetic tree. As shown in Figure 4, all six species occur in south and southeast China, with their distributional ranges adjacent to each other or sometimes even with minor overlap. For a detailed comparison of the six species, see Table 2.
|
|
|
|
Sinosenecio jiangxiensis is readily distinguished from S. jiuhuashanicus by its stem leafless, or with only a single bract-like leaf near the middle, and thus somewhat scapiform (vs. stem leafy with 3-7 cauline leaves similar to radical leaves). It should be noted that S. jiuhuashani-cus is a variable species in which two geographical races with some morphological differentiation can be distinguished. The populations in southern Anhui and eastern Jiangxi (Nanfeng County) have terete stem, auricles of cauline leaves becoming larger upward, and the uppermost leaves sometimes sessile with the lamina confluent with the auricle (Figure 11A); those in northern to western Jiangxi and eastern Hunan often have a trigonous stem, auricles not becoming larger or sometimes even becoming smaller upward, and the uppermost leaves petiolate with the lamina not confluent with auricle (Figure 11B). We included in our phylogenetic analysis one accession (Ying Liu 2008020) of the former race and two (Ying Liu & Tao Deng 2008018, 2008019) of the latter. As shown in Figure 9, accessions from the same race formed a well supported clade (PP = 1.00; BS= 99% and 100%), whereas
|
|
|
|
Among the six related species, Sinosenecio jiangxien-sis, S. saxatilis and S. wuyiensis are rather locally endemic (Figure 4; Table 2), and are morphologically also easily distinguishable from each other, even though S. wuyiensis is one of the closest relatives of S. jiangxiensis, as revealed in our ITS phylogenetic analysis. Sinosenecio saxatilis (Figure 10A) differs from S. jiangxiensis in the leaf lamina regularly palmately 5(-7)-lobed with ovate-triangular lobes (vs. dentate or shallowly lobed with broad, somewhat rounded mucronulate teeth or lobes). Sinosenecio
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 10. Sinosenecio saxatilis (A) and S. wuyiensis (B), showing their habit and leaf shape (A from Ruyuan, Guangdong, vouched by Ying Liu 2008025, IBSC, PE; B from Wuyi Shan, Fujian, vouched by Ying Liu 2008014, IBSC, PE).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIU and YANG ― Sinosenecio jiangxiensis, a new species from China
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 11. Sinosenecio jiuhuashanicus (A, B) and S. latouchei (C-E). A, S. jiuhuashanicus, showing the uppermost cauline leaves with the lamina confluent with auricle (from Huang Shan, Anhui, vouched by Ying Liu 2008020, IBSC, PE); B, S. jiuhuashanicus, showing the uppermost cauline leaves with the lamina not confluent with auricle (from Pingxiang, Jiangxi, vouched by Ying Liu & Tao Deng 2008018, IBSC, PE); C-E, S. latouchei, showing habit (C), cauline leaf (D), and involucre ecalyculate (E) (from Nanping, Fujian, vouched by Ying Liu & Tao Deng 2008010, IBSC, PE).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Figure 12. Sinosenecio guangxiensis. A, China, Guangxi, Chuen Yuen, T. S. Tsoong 83302 (A), holotype of S. guangxiensis; B, China, Guangxi, Xingan, Guangxi Exped. 4257 (PE); C, Habit; D, Capitula (bottom view, showing phyllaries and bracteoles); E, Petiole base (indicated by arrow) not auriculate (C-E from Xingan, Guangxi, vouched by Ying Liu 2008021, IBSC).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIU and YANG ― Sinosenecio jiangxiensis, a new species from China
|
|
|
|
|
|
|
|
|
the lineage composed of accessions belonging to different races received only weak support (PP = 0.58; BS= 58% and 54%). The significance of this geographical variation remains to be assessed.
|
dentate or palmately lobed to 1/2, lobes apically 2-
or 3-denticulate, both surfaces glabrous ................
............................................................ S. wuyiensis
4. Leaf lamina reniform or suborbicular, regularly 5-7-palmatilobed, lobes ovate-triangular, both surfaces glabrous or sometimes white tomentose abaxially and later glabrescent.............. S. saxatilis
3. Ovaries and achenes pubescent.
5. Stem erect or flexuous; cauline leaves 1-3; leaf lamina adaxially villous with spreading hairs; leaf auricles 4-10 mm in diameter ..............S. latouchei
5. Stem erect; cauline leaves 3-7; leaf lamina adaxi-ally pubescent with appressed hairs or sparsely or densely white tomentose; leaf auricles 7-30 mm in diameter ..................................... S. jiuhuashanicus
|
|
|
|
Owing to their similarity in posture, leaf shape, abaxial leaf lamina indumentum and achene pubescence, some plants of Sinosenecio jiangxiensis are morphologically similar to the extreme variants of S. latouchei with smaller stature and with only one reduced, bract-like cauline leaf. The two species are distinguished primarily by the color and indumentum of the adaxial surface of the leaf lamina, in the presence or absence of a calyculus, and in plant and leaf size (Table 2). In S. jiangxiensis, the adaxial surface of the leaf lamina is dark green and sparsely appressed-pu-bescent, the involucres are somewhat calyculate, the plants are 7.5-20 cm tall, and the leaves are 0.7-2.1(-3) x 1.0-2.7(-4) cm in size (Figure 3). In contrast, the leaf lamina of S. latouchei is adaxially green and villous with spreading hairs, the involucres are ecalyculate, the plants are 15-35 cm tall, and the leaves are 2.5-5 x 3-6 cm in size (Figure 11C-E). The two species also have independent geographical ranges and different habitat preferences (Table 2).
|
|
|
|
Acknowledgments. We are very grateful to Dr. David E. Boufford for his invaluable comments on the manuscript. We thank Ms. Wang Xiao-lan and Mr. Deng Tao for their help in the field, and Ms. Liu Yun-xiao for preparing the illustration. This work was supported by the National Natural Science Foundation of China (Grant no. 31100160).
|
|
|
|
|
|
|
|
Sinosenecio guangxiensis is a highly variable species in plant size, leaf size, indumentum of leaf lamina, and number of capitula (Figure 12A-B) (also see Jeffrey and Chen, 1984; Chen et al., 2011). Sinosenecio jiangxiensis resembles smaller statured variants of S. guangxiensis with smaller leaf lamina abaxially densely white tomentose, and with fewer capitula. In particular, the presence of a calyculus in both species (Figures 3C, 12D), a character absent in other species of Sinosenecio, sometimes results in misidentifications. Sinosenecio jiangxiensis, however, is distinguishable from S. guangxiensis in the stem often leafless or with only 1 bract-like leaf near the middle, and the petiole of the single cauline leaf (when present) slightly auriculate at the base (Figure 3). Sinosenecio guangx-iensis has 1-5 cauline leaves similar to the radical ones, and the petioles are never auriculate at the base (Figure 12A-E). Geographically, the two species also are independent (Figure 4; Table 2). In our ITS phylogenetic analysis, S. guangxiensis and S. jiangxiensis are nested within two independent lineages, indicating their distinction at species level.
|
|
|
|
|
Chen, Y.L., Y. Liu, Q.E. Yang, B. Nordenstam, and C. Jeffrey. 2011. Sinosenecio B. Nord. In Wu Z.Y. and P.H. Raven (eds.), Flora of China Volume 20-21. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, pp. 464481.
Doyle, J.J. and J.L. Doyle. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19: 11-15.
Guindon, S. and O. Gascuel. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52: 696-704.
Hall, T.A. 1999. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symp. Ser. 41: 95-98.
Huelsenbeck, J.P. and F.R. Ronquist. 2001. MRBAYES: Bayes-
ian inference of phylogenetic trees. Bioinformatics 17: 754755.
Jeffrey, C. and Y.L. Chen. 1984. Taxonomic studies on the tribe Senecioneae (Compositae) of Eastern Asia. Kew Bull. 39:
205-446.
Levan, A., K. Fredga, and A.A. Sandberg. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52:
201-220.
Liu, J.Q. 1999. Systematics of the tribe Senecioneae subtribe Tussilagininae (Asteraceae) of the Eastern Asia. Ph.D. thesis, Institute of Botany, Chinese Academy of Sciences, Beijing.
Liu, Y. 2010. Systematics of the genus Sinosenecio B. Nord. (Asteraceae). Ph.D. thesis, Institute of Botany, Chinese Academy of Sciences, Beijing.
|
|
|
|
Key to Sinosenecio jiangxiensis and its related species
|
|
|
|
1. Involucres calyculate.
2. Cauline leaf absent or 1 and bract-like; base of petiole of cauline leaf slightly auriculate; capitula solitary, rarely 2 or 3...........................S.jiangxiensis
2. Cauline leaves 1-5, similar to radical ones; base of petiole of cauline leaves never auriculate; capitula 1-7 or more................................ S. guangxiensis
1. Involucres ecalyculate.
3. Ovaries and achenes glabrous.
4. Leaf lamina broadly flabellate or suborbicular,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Botanical Studies, Vol. 53, 2012
|
|
|
|
|
|
|
|
Liu, Y. and Q.E. Yang. 2011a. Cytology and its systematic implications in Sinosenecio (Senecioneae-Asteraceae) and two closely related genera. Pl. Syst. Evol. 291: 7-24.
Liu, Y. and Q.E. Yang. 2011b. Floral micromorphology and its systematic implications in the genus Sinosenecio (Sene-cioneae-Asteraceae). Pl. Syst. Evol. 291: 243-256.
Liu, Y. and Q.E. Yang. 2011c. Sinosenecio sichuanicus (Aster-aceae), a new species from Sichuan, China. Bot. Stud. 52:
219-223.
Nordenstam, B. 1978. Taxonomic studies on the tribe Sene-cioneae (Compositae). Opera Bot. 44: 1-84.
Nordenstam, B. and P.B. Pelser. 2011. Notes on the generic limits of Sinosenecio and Tephroseris (Compositae-Senecione-ae). Comp. Newsl. 49: 1-7.
Nordenstam, B., P.B. Pelser, J.W. Kadereit, and L.E. Watson. 2009. Senecioneae. In V.A. Funk, A. Susanna, T.F. Stuessy,
|
and R.J. Bayer (eds.), Systematics, Evolution, and Bioge-ography of Compositae. International Association for Plant Taxonomy, Vienna, pp. 503-525.
Posada, D. and K.A. Crandall. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817-818.
Swofford, D.L. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), version 4.0b10. Sinauer Associates, Sunderland.
Wang, L.Y., P.B. Pelser, B. Nordenstam, and J.Q. Liu. 2009.
Strong incongruence between the ITS phylogeny and generic delimitation in the Nemosenecio-Sinosenecio-Tephroseris assemblage. Bot. Stud. 51: 435-442.
White, T.J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. Innis, D. Gelfand, J. Sninsky, and T.J. White (eds.), PCR Protocols: A Guide to Methods and Applications. Academic Press, San Diego, pp. 315-332.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1中山大學生命科學學院
2中國科學院植物資源保護與可持續利用重點實驗室(華南植物園)
|
|
|
|
|
|
|
|
本文描述了中國江西產蒲兒根屬一新種:江西蒲兒根(Sinosewecz'o jiangxiensis Y. Liu & Q. E.
Yang )。其體細胞染色體數目為2n = 48 。核型公式為2n = 42m + 4sm + 2st(2sat)。本新種在葉形、葉背毛
被和瘦果毛被方面與廣西蒲兒根(S. guangxiensis C. Jeffrey & Y. L. Chen)以及白背蒲兒根(S. latouchei
(J. F. Jeffrey) B. Nord.)相似,但以植株和葉片均較小,莖葶狀,無葉或僅在近中部具1枚苞片狀葉,頭
狀花序通常1而與後兩者相區別。利用ITS序列進行的系統發育分析表明,江西蒲兒根與廣西蒲兒根、
九華山蒲兒根(S. jiuhuashanicus C. Jeffrey & Y. L. Chen)、白背蒲兒根、岩生蒲兒根(S. saxatilis Y. L.
Chen)以及武夷山蒲兒根(S. wuyiensis Y. L. Chen)相近,可能與九華山蒲兒根、白背蒲兒根以及武夷
山蒲兒根最為近緣。本文詳細比較了這些種類的特徵並提供了分種檢索表。
|
|
|
|
|
|
|
|
關鍵詞:菊科;染色體數目;核型;千里光族;江西蒲兒根。
|
|
|
|
|
|
|
|
|
|
|