Bot. Bull. Acad. Sin. (1996) 37: 51-59
Roberts et al. — Nitrate reductase during shoot morphogenesis
In vitro tobacco cultures provide a convenient system for
investigating nitrate reductase regulation during shoot
morphogenesis
M.A. Roberts1, M.P. Watt2,4, and B.I. Huckett3
1School of Biological and Medical Sciences, University of St Andrews, St Andrews, KY 16 9th FIFE, UK
2Biology Department, University of Natal, Private Bag X10, Dalbridge 4014, South Africa
3Biotechnology Department, SA Sugar Association Experiment Station, Private Bag X02, Mount Edgecombe 4300, South Africa
(Received June 13, 1995; Accepted October 26, 1995)
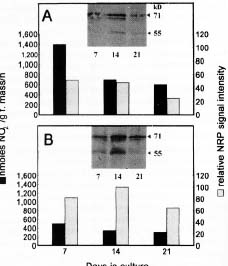
Abstract. Nicotiana tabacum callus culture was assessed in terms of its usefulness as a system for investigating the regulation of nitrate reductase (NR; EC 1.6.6.1) during shoot morphogenesis. Culture conditions for organogenesis and appropriate methodologies for extraction and assay of NR during callus growth and development were established. The most suitable medium comprised Murashige and Skoog salts and vitamins, sucrose, indoleacetic acid and kinetin. Provided that NO3_-N was present, callus growth and shoot morphogenesis occurred in the same culture step. Optimised in vivo, in situ, and in vitro NR assays yielded similar values and patterns during culture development, and the in vivo assay was selected for subsequent studies on the basis of ease and efficiency of use. With quantitative alteration in nitrogen supply (60 vs. 120 mM; 1:2 NH4+-N : NO3_-N) and variations in light regime (16 /8 h light/dark vs. continuous darkness), callus growth parameters were comparable in terms of nitrate uptake, increase in fresh mass and protein accumulation. However, a marked rise in the level of in vivo NR activity (NRA) was observed just prior to shoot primordia emergence in light/dark-grown but not in dark-grown calli. In protein blot analyses using a polyclonal antibody raised against spinach NR, major protein bands representing holo-NR cleavage products were identified at 71 and 55 kDa. Putative nitrate reductase protein (NRP) levels were compared with in vivo NRA during the final stages of callus differentiation under both light/dark and continuous dark conditions. NRP variations appeared to be much less marked than those of NRA, suggesting the possibility that post-translational modification is a significant feature of NR regulation. The potential value of the system for further studies of NR regulation at the molecular level is discussed.
Keywords: Callus; Differentiation; Morphogenesis; Nitrate reductase; Nitrate reductase assays; Tobacco.
Introduction
Nitrate reductase (NR; EC 1.6.6.1) is considered to be a limiting factor for higher plant growth, development and protein production, and much research has been undertaken involving the delineation of the regulatory properties of this enzyme (e.g. Solomonson and Barber, 1990) due to the potential for increasing nitrogen assimilation efficiency. Regulation of NR is achieved by several sensitive and complex control systems, with precise regulating mechanisms differing among higher plant species and responding variously to cellular conditions (Beevers and Hageman, 1969; Caboche et al., 1989; Campbell, 1989). Nitrate reductase activity (NRA) is known to change during organ and plant development (Kenis et al., 1992), as do the activities of other enzymes involved in the nitrate assimilation pathway (de la Haba et al., 1988). Nevertheless, much further work needs to be done on the mechanisms involved in developmental NR regulation in higher plants, including the possible roles of this enzyme in cell and organ differentiation processes.
Cellular differentiation and morphogenesis arise from progressive developmental interactions between cells and their environment, leading to the initiation of activity of specific genes, which may be regulatory or required for enhanced metabolism (Gyorgyey et al., 1991). The influence of certain endogenous factors (e.g. phytohormone levels) (Li et al., 1992) and environmental influences (e.g. light, temperature) (Thorpe, 1983; Tran Thanh Van and Trinh, 1990) on these processes is undisputed. Yet, as higher plant differentiation is difficult to decipher at the whole plant level due to numerous biological constraints (Tran Thanh Van and Trinh, 1990), research with whole plants has been restricted largely to descriptive analyses rather than the elucidation of causal mechanisms.
In vitro plant culture systems have been developed that facilitate morphological, physiological, cytological, biochemical and molecular work on differentiation (Tran Thanh Van and Trinh, 1990). Such cultures approximate the responses of their whole plant counterparts genetically and morphologically, and are highly suitable for growth and differentiation studies in terms of generation of material at the required stage of morphogenesis and manipulation of nutritional and environmental parameters (Bisbis
4Corresponding author.