Bot. Bull. Acad. Sin. (1996) 37: 121-126
Yang et al. — Pigment and surfactant
Pigment solubilization of the chloroplast thylakoid membranes by a surfactant
Chi-Ming Yang1, Jen-Chieh Hsu, Yih-Kuang Lu, and Ming-Horng Yin
Institute of Botany, Academia Sinica, Nankang, Taipei, Taiwan 115, Republic of China
(Received February 13, 1995; Accepted January 29, 1996)
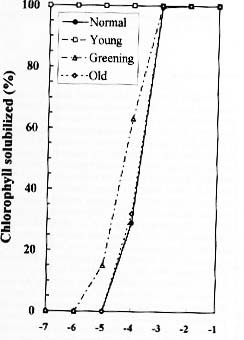
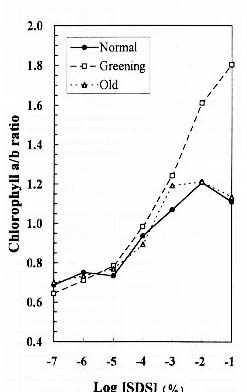
Abstract. We examined the influence of various concentrations of sodium dodecyl sulfate (SDS) on the pigment solubilization of the chloroplast thylakoid membranes isolated from leaves at different developing stages of normal and Golden-leaves figs (Ficus microcarpa). When the SDS concentration was lower than 10-5 %, more carotenoids, especially neoxanthin, were released than chlorophyll (Chl), and more Chl b was released than Chl a. When SDS concentration was between 10-5 % and 10-3 %, more Chl molecules were released than carotenoids. As the SDS concentration was increased above 10-3 %, all Chl molecules and carotenoids were solubilized. Among the solubilized carotenoids, the release sequence from thylakoid membrane may be neoxanthin, antheraxanthin, (lutein, violaxanthin, taraxanthin, b-carotene), a-carotene, and zeaxanthin. We conclude that in the pigment-protein complexes, carotenoids (especially neoxanthin) are more susceptible to SDS than Chl molecules, that Chl b is more susceptible to SDS than Chl a, and that neoxanthin may be directly exposed to the stroma face of the thylakoid membrane.
Keywords: Carotenoid; Chlorophyll; Neoxanthin; Pigment-protein complex; Release sequence; Solubilization; Surfactant; Thylakoid membrane.
Introduction
Surfactants have been widely used to study the organization and constituents of biological membranes (Helenius and Simons, 1975; Rosen, 1978). Much of our current knowledge about the structure and molecular organization of the higher plant photosynthetic apparatus is derived from studies using surfactants. The isolation of pigment-protein complexes from higher plant chloroplasts was performed by the aid of surfactants (Markwell et al., 1978; Markwell, 1986). Many surfactant systems developed to fractionate photosynthetic pigment-protein complexes, however, may not fully solubilize the complexes prior to the electrophoretic step (Allgood et al., 1991). The lateral distribution of thylakoid membrane components was obtained by differential solubilization of the chloroplast thylakoid membranes with surfactants (see refs. of Markwell and Thornber, 1982; Bartzatt et al., 1983). Many photochemical activities of photosynthetic apparatus are dramatically altered after surfactant treatment (Apostolova and Ivanov, 1995). Although it has been reported that the photosynthetic apparatus of one organism Dunaliella teriolecta appears to be unusually sensitive to surfactant Triton X-100 (Sukenik et al., 1989), the surfactant concentrations usually used in the litera
ture have produced similar results with most species. Chloroplast thylakoid protein phosphatase was monitored by Triton X-100 to be membrane surface-associated (Sun et al., 1989). Studies at sub-solubilizing concentrations have indicated that the primary interaction between the surfactant and the thylakoid membrane may involve adsorption at the membrane/solution interface rather than insertion of surfactant molecules into the membrane, and that surfactants may specifically interact with exposed PSI components on the surface of the thylakoid membrane (Bartzatt et al., 1983).
The perturbation of Chl molecules within pigment-protein complexes is a sensitive indicator of change in the local environment of thylakoid membranes. Little information is available about the selective effect of surfactants on the thylakoid membrane components. There have been no reports at all, however, on the selective or differential solubilization of Chl a and b and carotenoids from the thylakoid membrane. The aim of this research is to investigate the selective effect of surfactants on the pigment solubilization of the chloroplast thylakoid membrane in higher plants.
Materials and Methods
Plants
About one-year-old normal and Golden-leaves figs (Ficus microcarpa cv. Golden-leaves) about 50 cm in height were purchased from a local nursery and grown for 6 weeks in a soil-vermiculite mixture in a greenhouse under natural light in the summer.
1Corresponding author. Fax: 886-2-7827954.