Bot. Bull. Acad. Sin. (2003) 44: 267-273
Lee et al. — Yam tuber mucilage exhibited ACE inhibitory activities
The mucilage of yam (Dioscorea batatas Decne) tuber exhibited angiotensin converting enzyme inhibitory activities
Mei-Hsien Lee1, Yin-Shiou Lin1, Yaw-Huei Lin2,*, Feng-Lin Hsu1, and Wen-Chi Hou1,*
1Graduate Institute of Pharmacognosy, Taipei Medical University, Taipei, Taiwan 110
2Institute of Botany, Academia Sinica, Nankang, Taipei, Taiwan 115
(Received July 29, 2003; Accepted September 12, 2003)
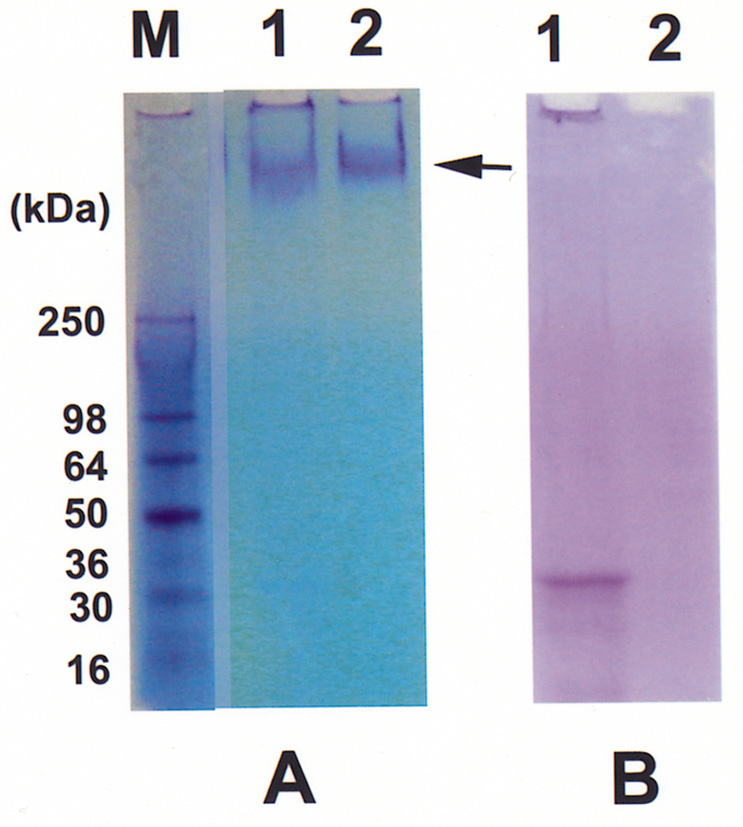
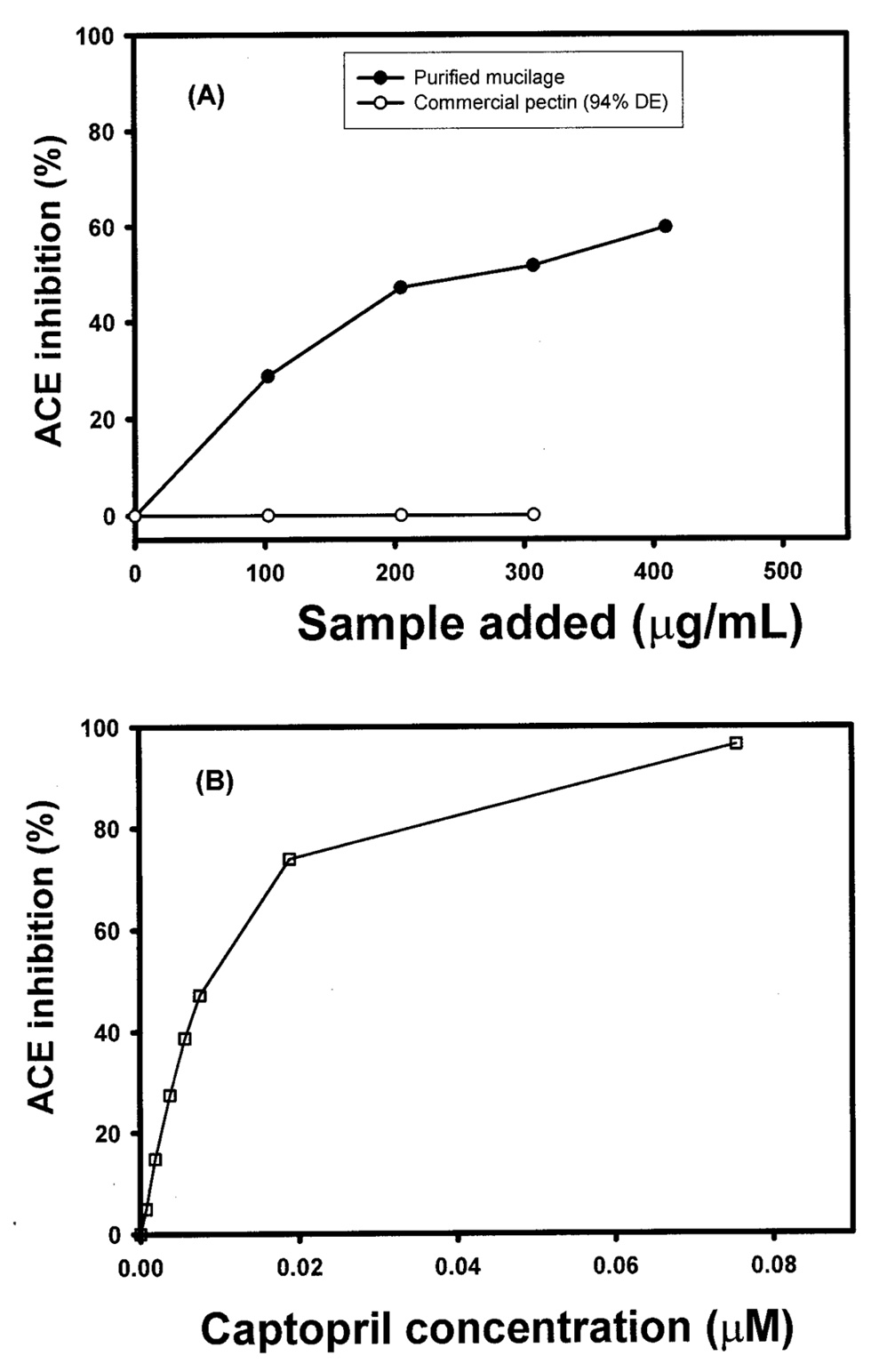
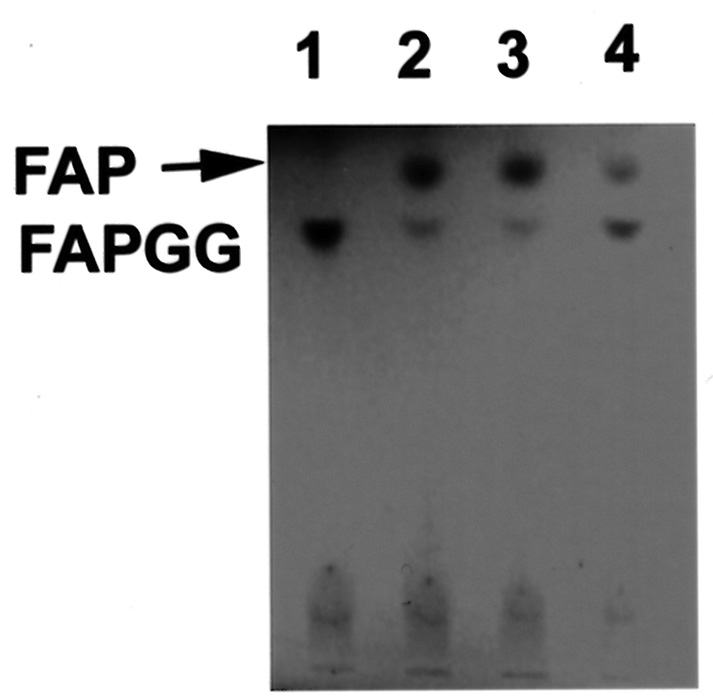
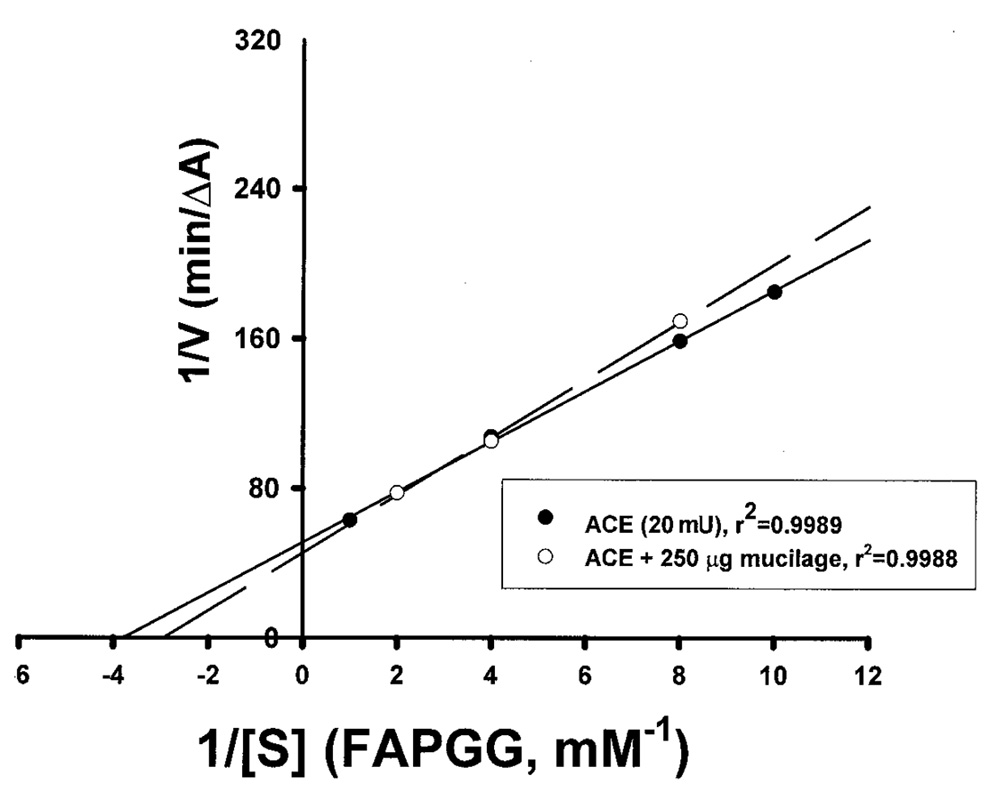
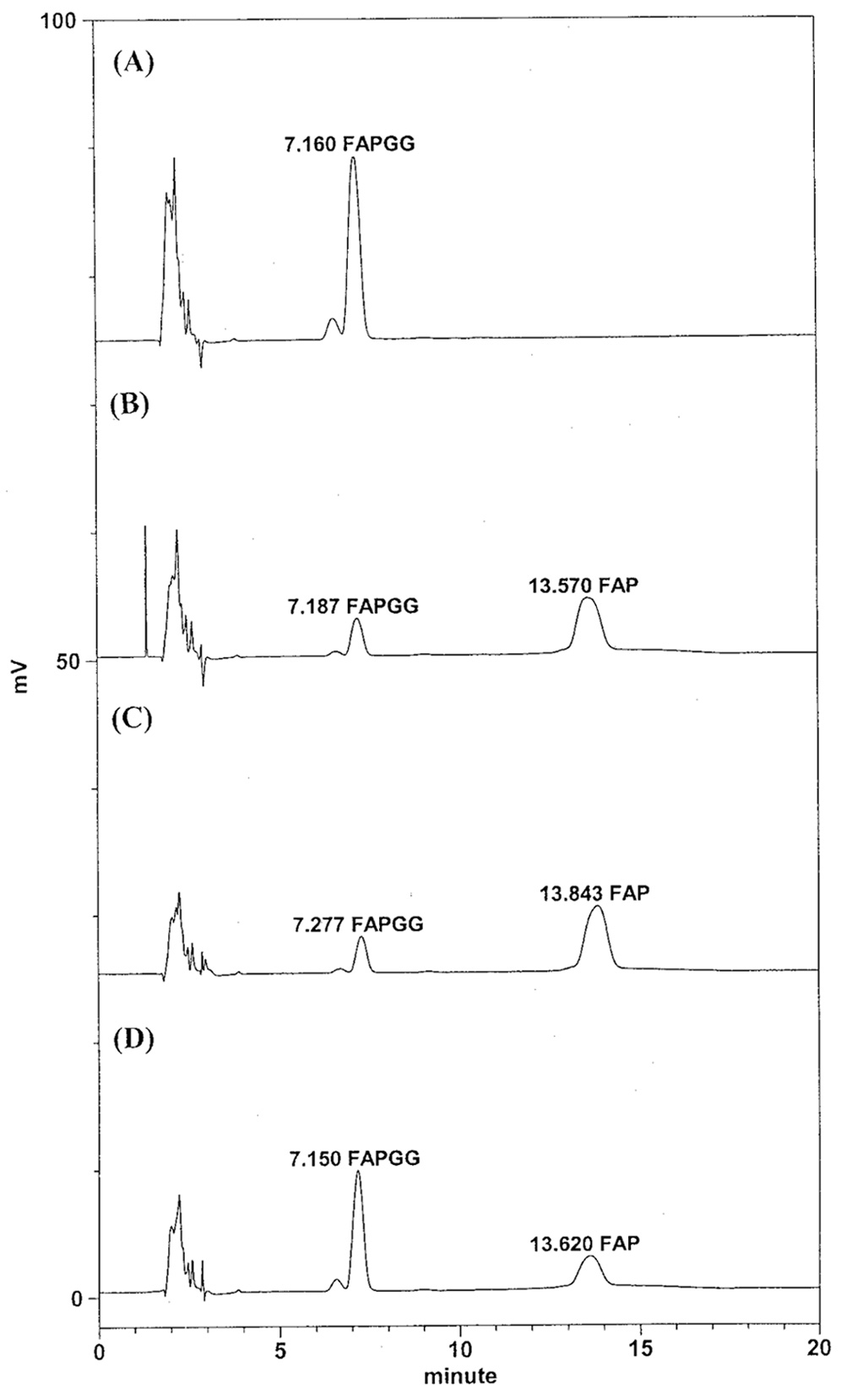
Abstract. The tuber mucilage of yam (Dioscorea batatas Decne) (YTM) was extracted and purified to homogeneity, which was confirmed by the toluidine blue staining on a sodium dodecylsulfate-polyacrylamide gel electrophoresis gel treated with 2-mercaptoethanol appearing as a single band with molecular mass larger than 250 kDa. This purified YTM was shown by spectrophotometric method to inhibit angiotensin converting enzyme (ACE) in a dose-dependent manner (28.7 to 59.8% ACE inhibition, respectively, by 102.46 to 409.84 µg/mL YTM) using (N-(3-[2-furyl]acryloyl)-P he-Gly-Gly) (FAPGG) as a substrate. The concentration of YTM required for 50% inhibition (IC50) of ACE activity was 256.2 µg/mL while that of captopril was 0.00781 µM (0.0095 nmole). The commercial polysaccharide pectin (102.46 to 307.38 µg/mL) showed no inhibitory activity against ACE. Using fluorescent silica TLC or C18 reverse phase HPLC to detect FAPGG and FAP, the results also showed that YTM inhibited ACE. The YTM showed mixed type inhibition against ACE, and the Michaelis constant in the presence of YTM was 0.33 mM. Consumption of yam tubers may benefit people's health.
Keywords: Angiotensin converting enzyme (ACE); HPLC; (N-(3-[2-furyl]acryloyl]-Phe-Gly-Gly) (FAPGG); Mucilage; TLC; Yam.
Introduction
Several risk factors are associated with stroke, including age, gender, elevated cholesterol, smoking, alcohol, excessive weight, race, family history, and hypertension (Mark and Davis, 2000). Although some of these risk factors cannot be modified, one factor that can be controlled and has the greatest impact on the etiology of stroke is high blood pressure (Dunbabin, 1992). Hypertension is considered to be the central factor in stroke with approximately 33% of deaths due to stroke attributed to untreated high blood pressure (Mark and Davis, 2000). Several classes of pharmacological agents have been used in the treatment of hypertension (Mark and Davis, 2000). One class of anti-hypertensive drugs, known as angiotensin I converting enzyme (ACE) inhibitors (ACEI, i.e. a peptidase inhibitor), are associated with a low rate of adverse side-effects and are the preferred class of anti-hypertensive agents when treating patients with concurrent secondary diseases (Fotherby and Panayiotou, 1999). ACE (peptidyldipeptide hydrolyase EC 3.4.15.1) is a dipeptide-liberating exopeptidase classically associated with the renin-angiotensin system regulating peripheral blood pressure (Mullally et al., 1996). ACE removes a dipeptide from the C-terminus of angiotensin I to form angiotensin II, a very hypertensive
compound. Several endogenous peptides, such as enkephalins, b-endorphin, and substance P, were reported to be competitive substrates and inhibitors of ACE (Mullally et al., 1996). Several food-derived peptides also inhibited ACE, including a-lactalbumin and b-lactoglobulin (Mullally et al., 1996), casein (Maruyama et al., 1987), zein (Yano et al., 1996), and gelatin (Chen et al., 1999; Kim et al., 2001). Several antioxidant peptides (reduced glutathione and carnosine-related peptides) (Hou et al., 2003) and synthetic peptides also exhibited ACEI activities (Chen et al., 2003).
Yam (Dioscorea species) is a member of the monocotyledonous family Dioscoreaceae and is a staple food in West Africa, Southeast Asia and the Caribbean (Akoruda, 1984). Yam is recognized as an herbal plant since dried tuber slices were frequently used as Chinese herbal medicines. The tuber storage proteins of yam, dioscorin, exhibited carbonic anhydrase and trypsin inhibitor activities (Hou et al., 1999a), both dehydroascorbate reductase and monodehydroascorbate reductase activities (Hou et al., 1999b), and antioxidant activities (Hou et al., 2001). Yam tuber contained mucilages reported to be a mannan-protein complex (Misaki et al., 1972; Tsai and Tsai, 1984). Recently, it was reported that yam tuber mucilage (YTM) exhibited antioxidant activities (Hou et al., 2002). In this work we report for the first time that purified YTM exhibits novel dose-dependent ACE inhibitory activities. Captopril was used as a positive control and commercial pectin as a negative control. The YTM showed mixed type inhibition against ACE, and the Michaelis constant in the presence of YTM was also determined.
*Corresponding author. Prof. Wen-Chi Hou, Phone: 886-2- 2736-1661 ext. 6160; Fax: 886-2-2378-0134; E-mail:wchou@tmu.edu.tw or Prof. Yaw-Huei Lin, Tel: 886-2-2789-9590 ext. 321; E-mail: boyhlin@ccvax.sinica.edu.tw