Bot. Bull. Acad. Sin. (2003) 44: 275-284
Ming et al. — Liquorice cell tolerance of water stress
Application of external calcium in improving the PEG-induced water stress tolerance in liquorice cells
Ming Li1, Gen-Xuan Wang1,2,*, and Jiou-Sheng Lin1
1State Key Laboratory of Arid Agroecology, Lanzhou University, 730000, Lanzhou, P.R. China
2School of Biology, Zhejiang University, 310027, Hangzhou, P.R. China
(Received October 30, 2002; Accepted May 14, 2003)
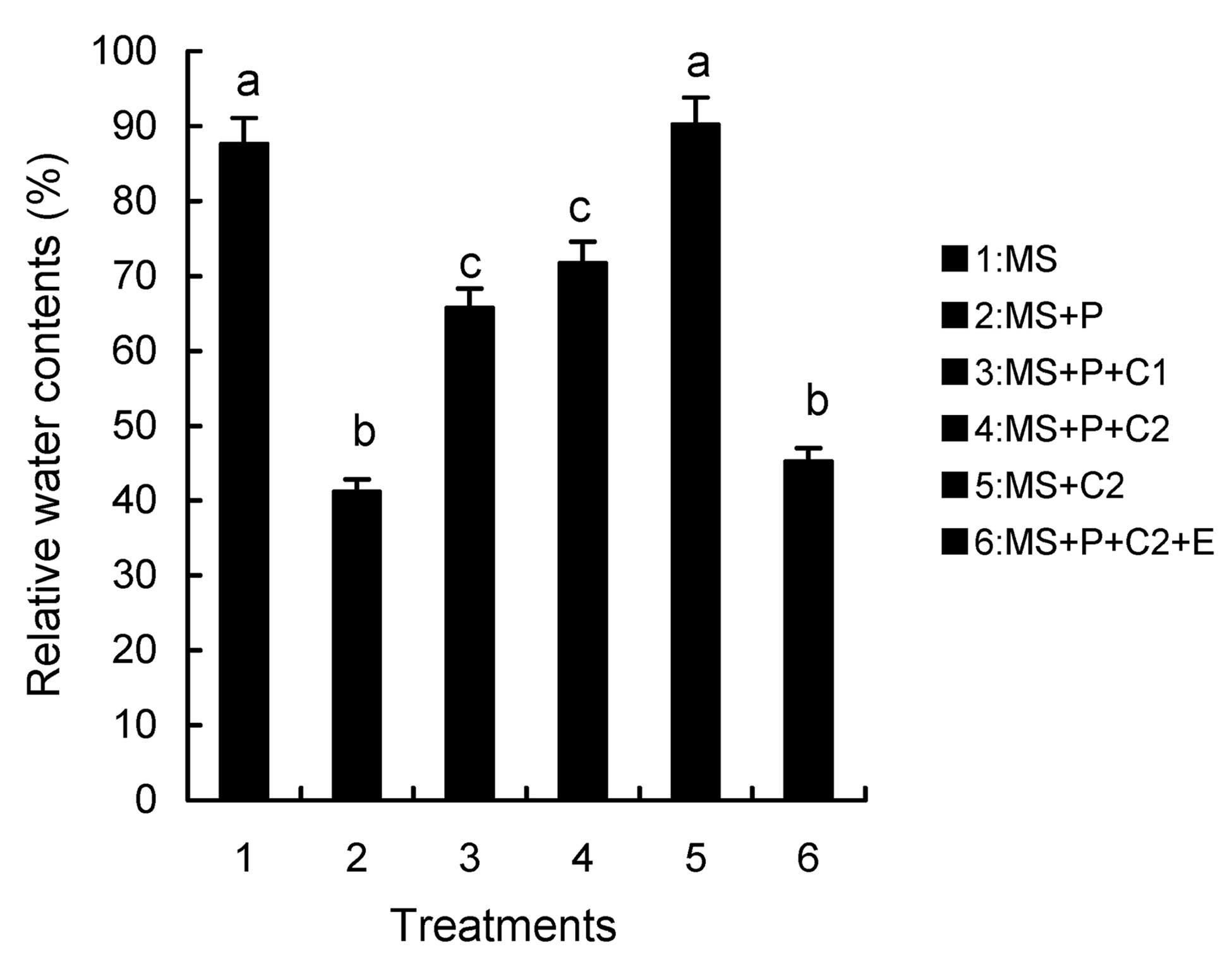
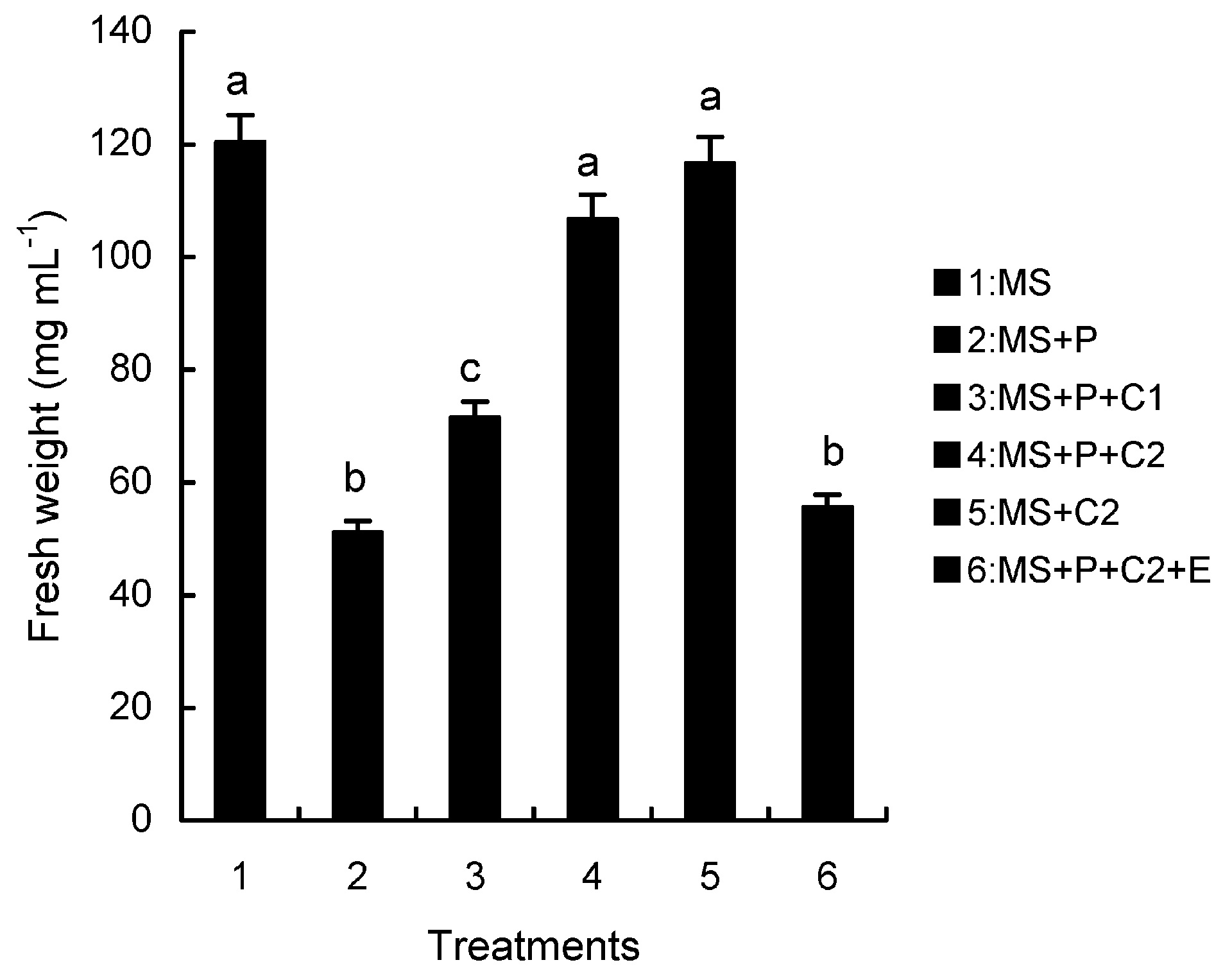
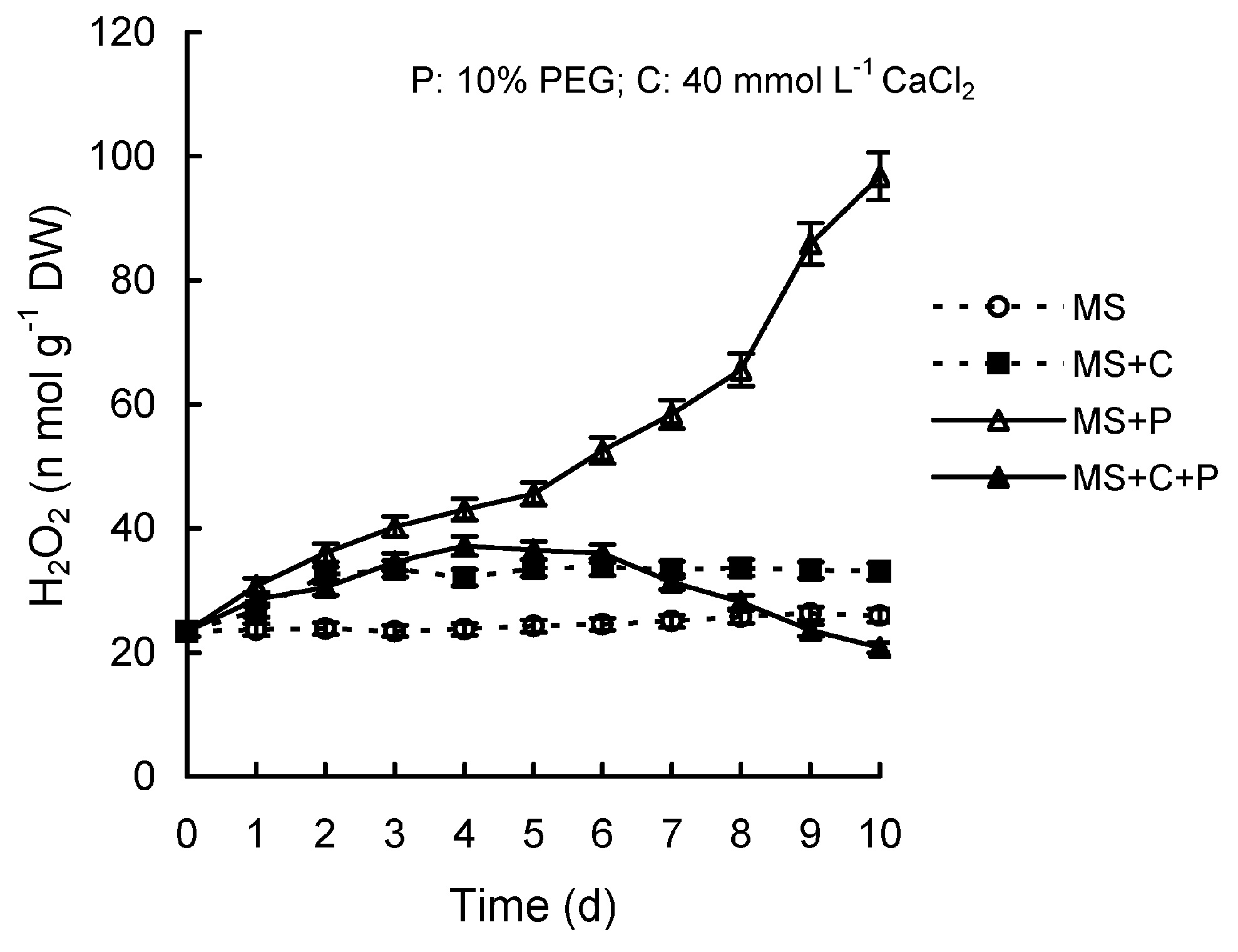
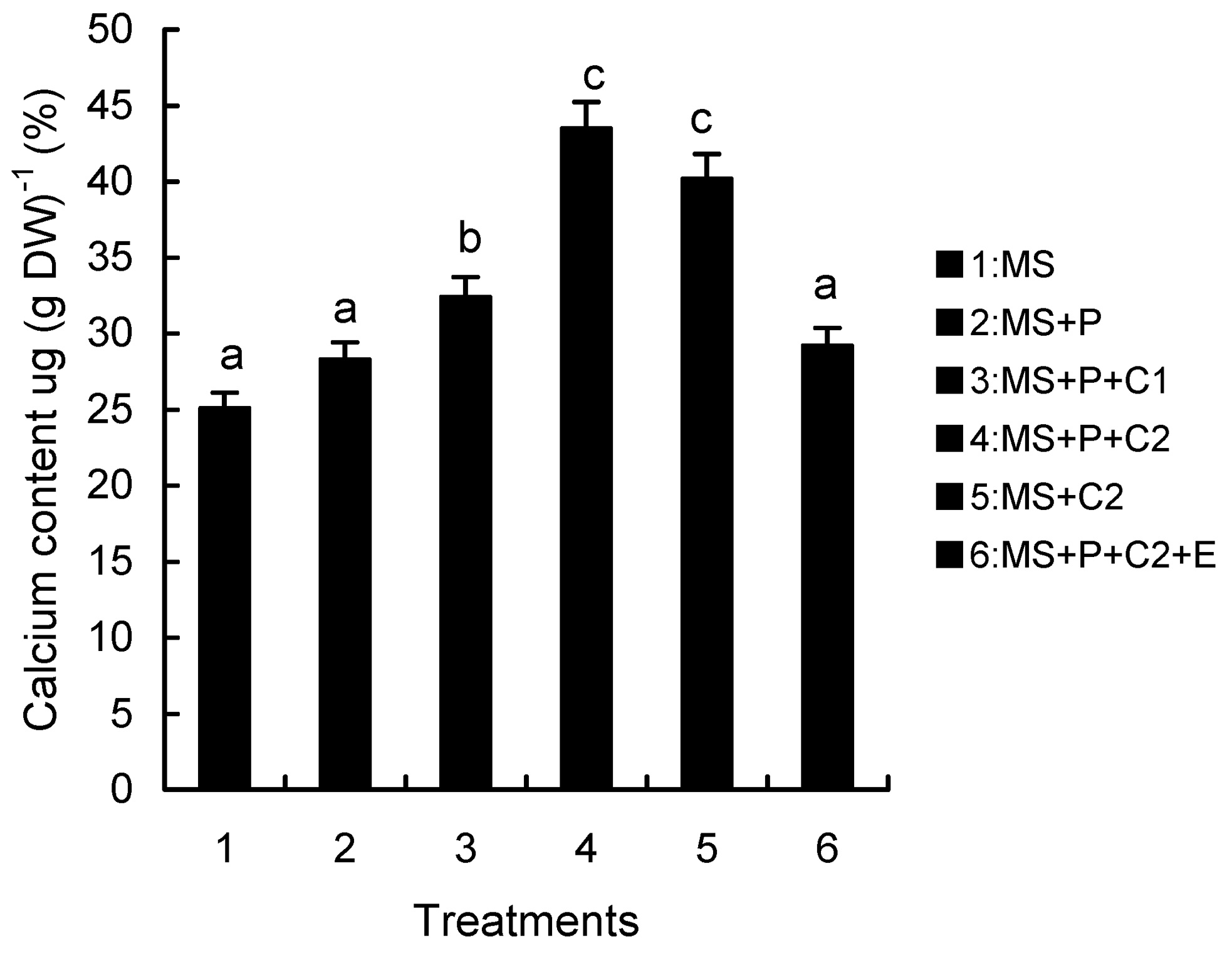
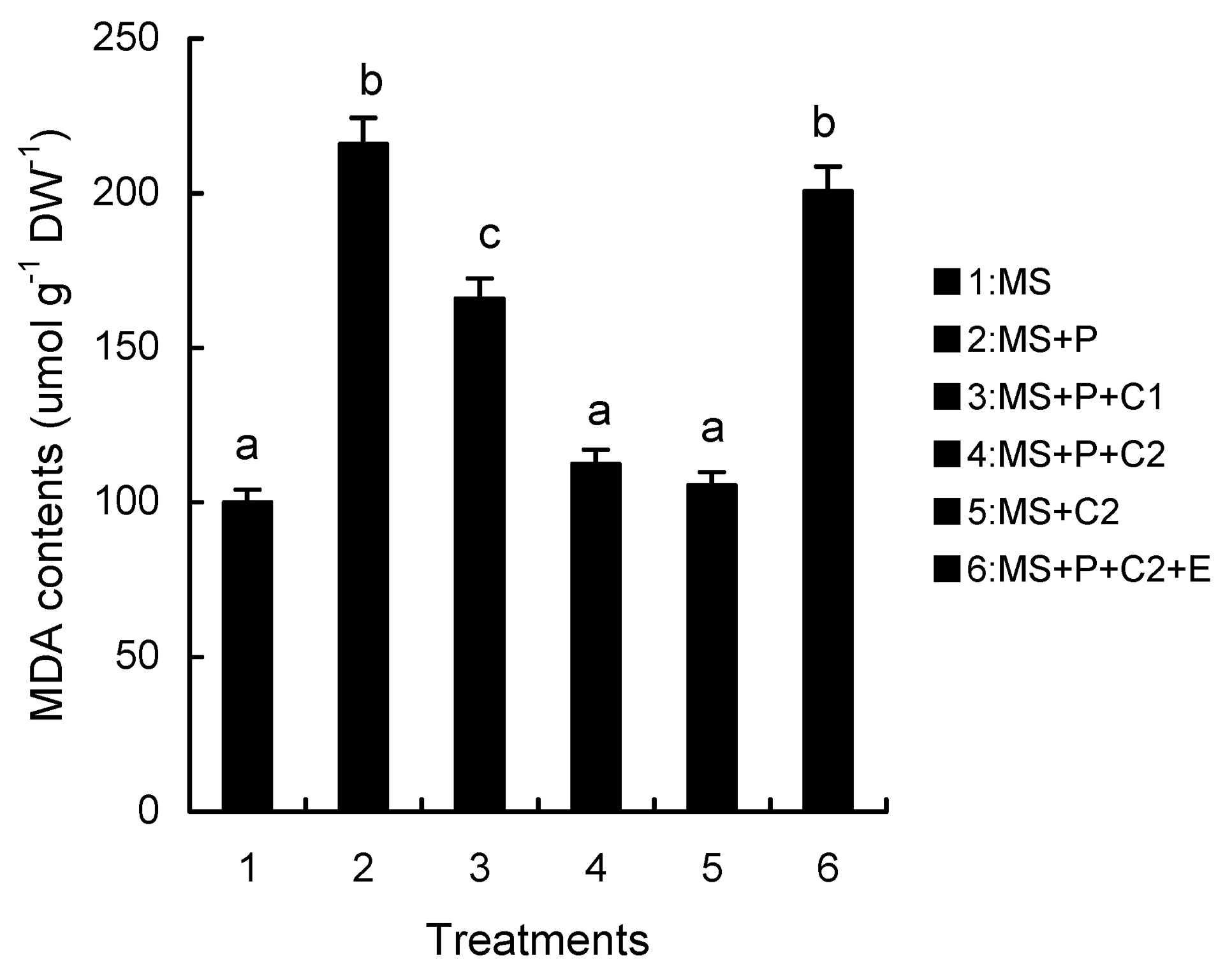
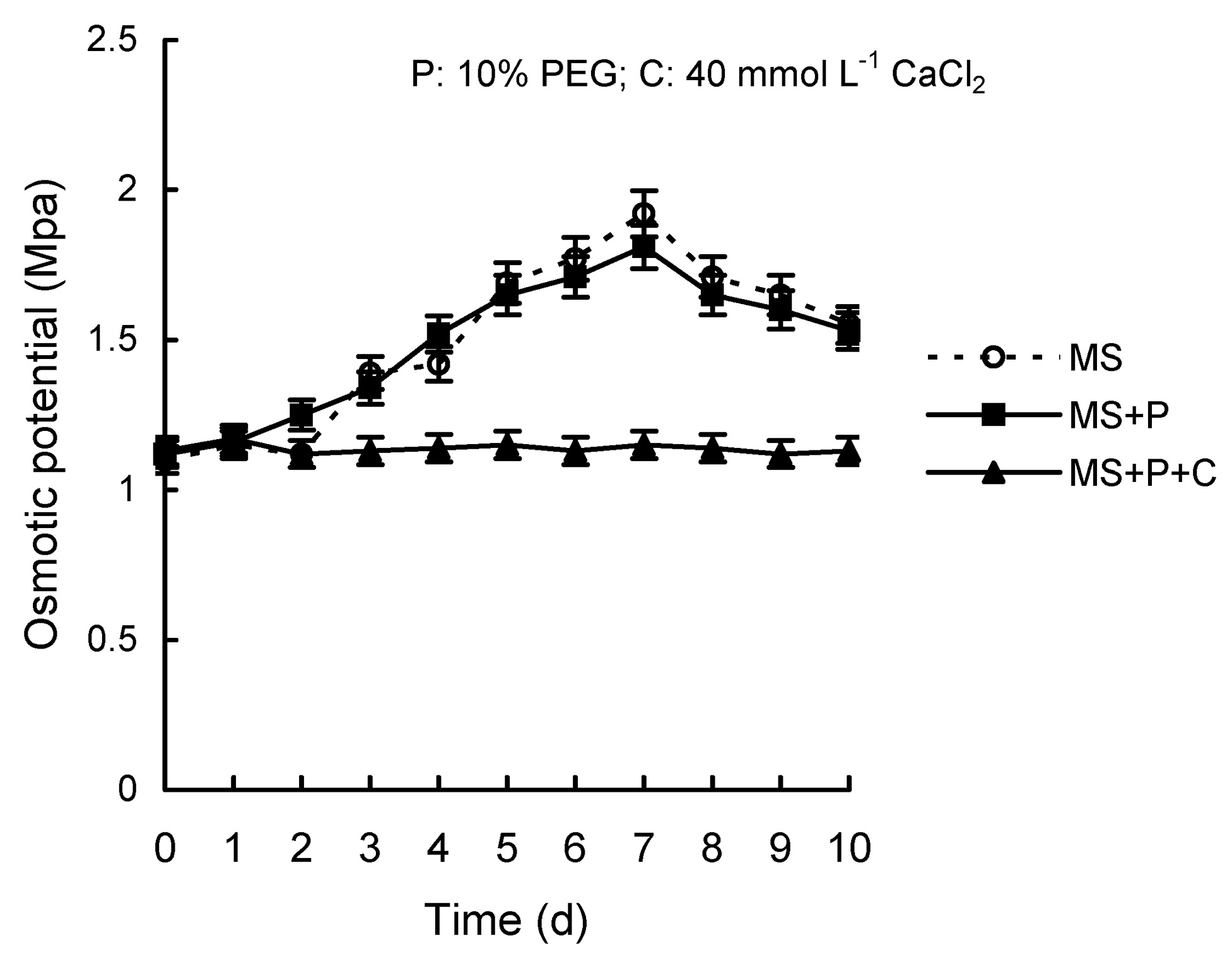
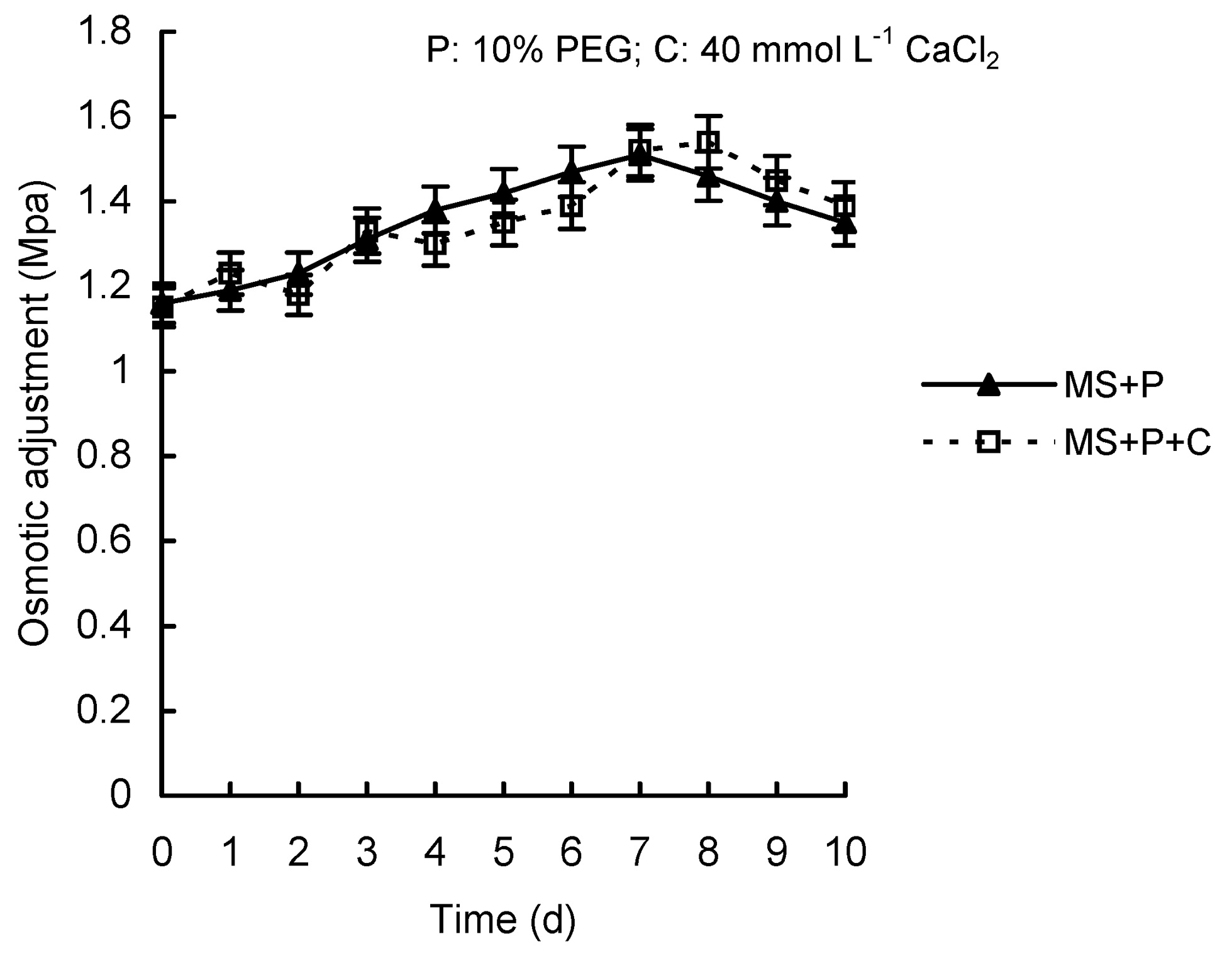
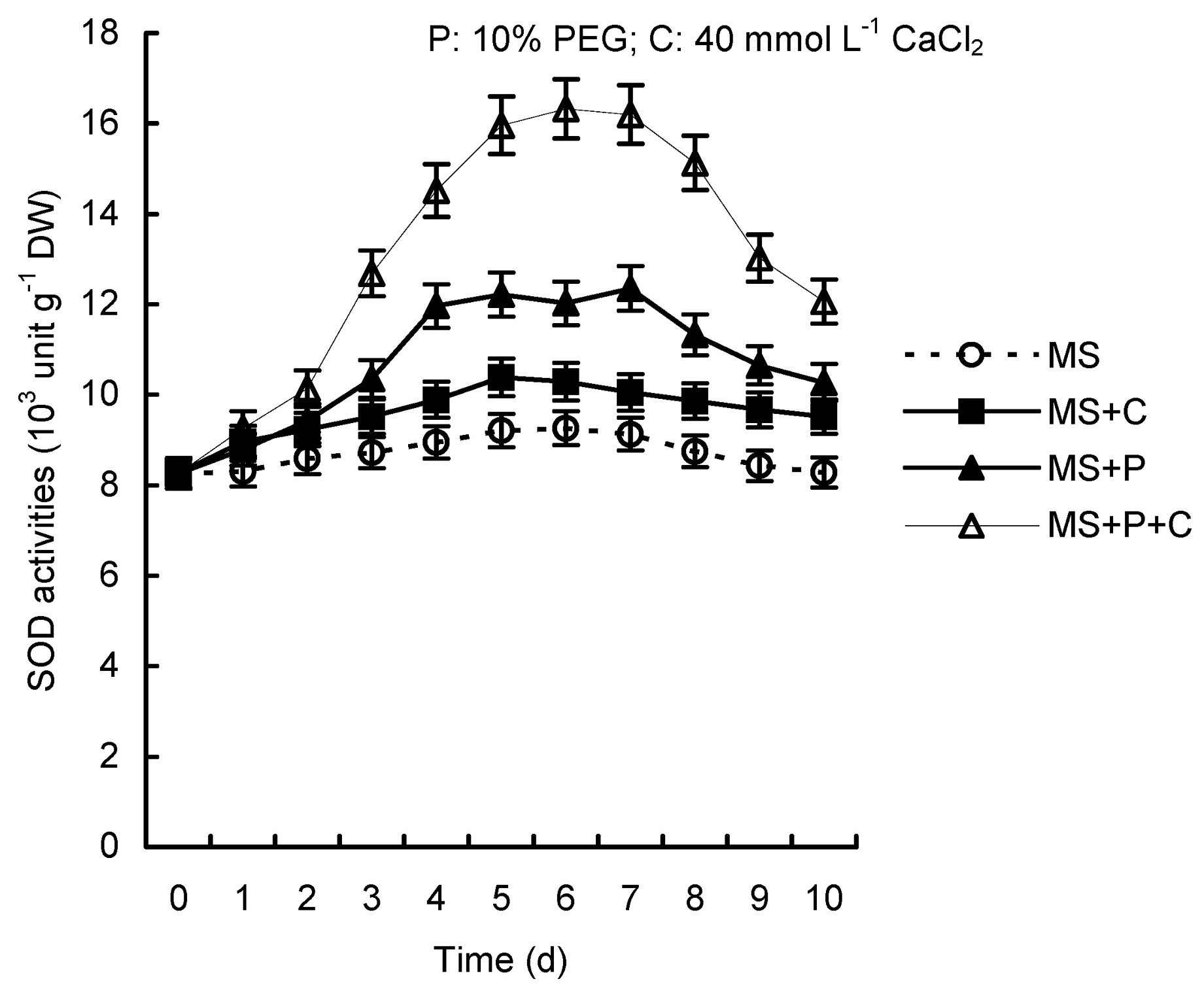
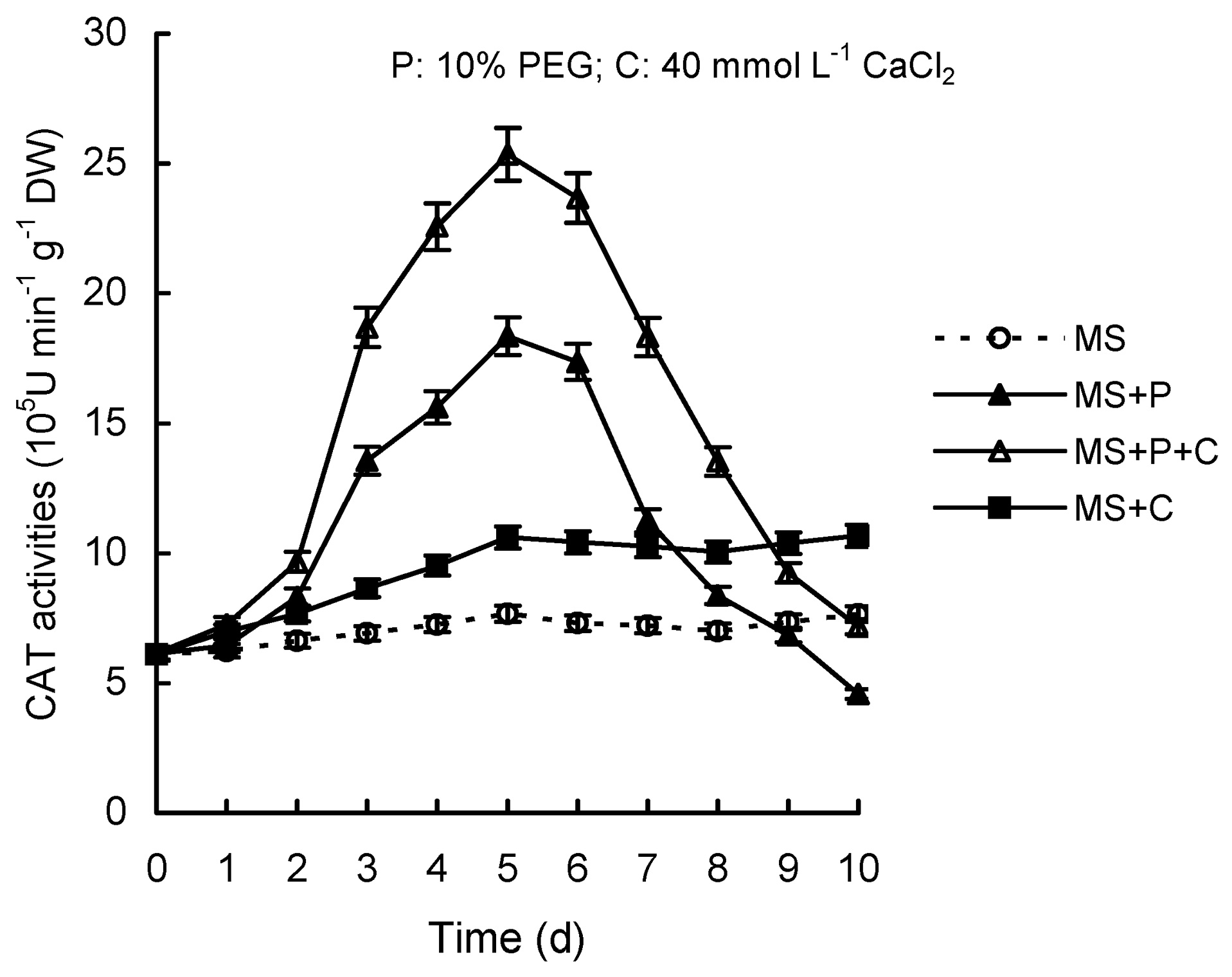
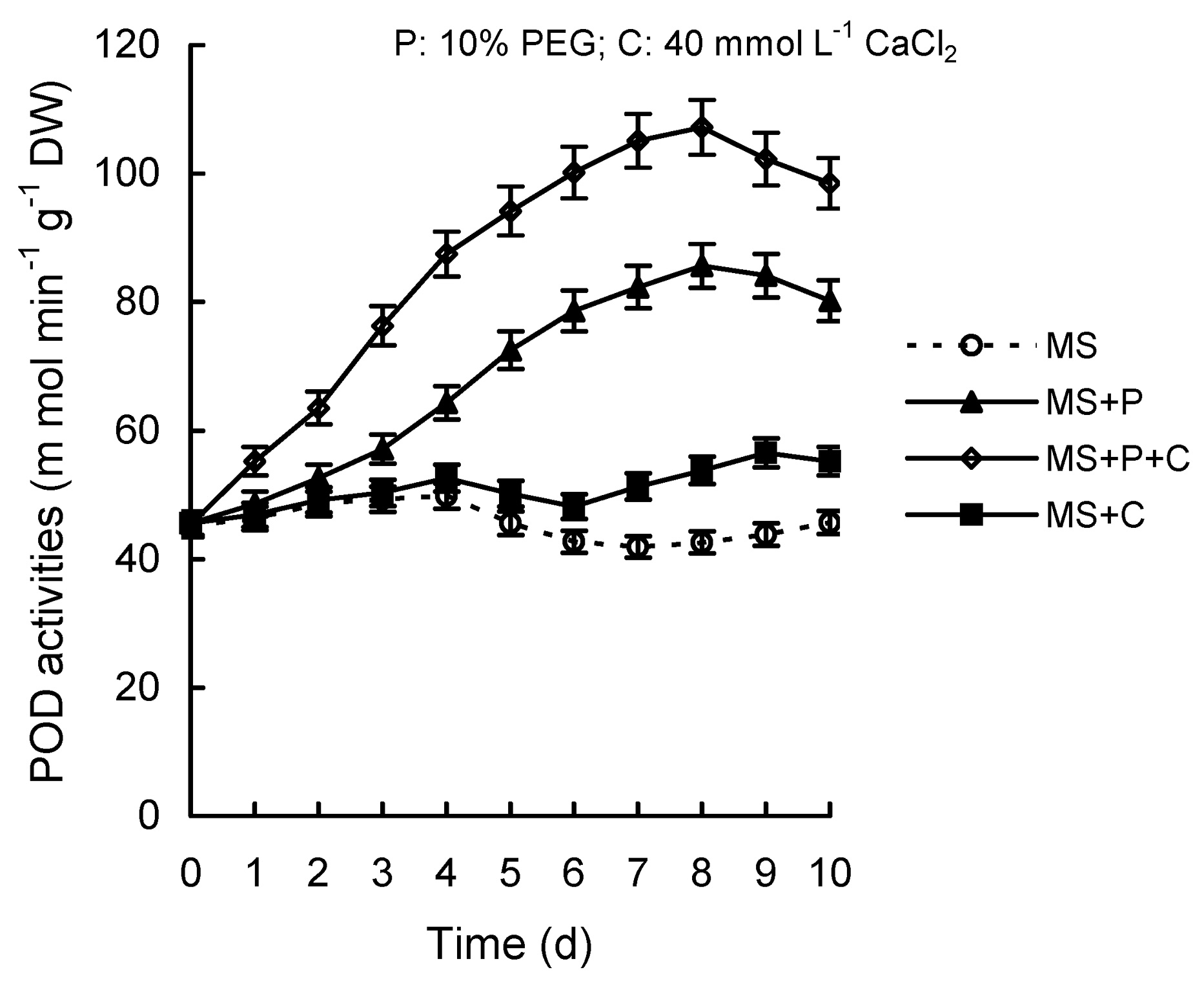
Abstract. Calcium (Ca2+) may be involved in plant tolerance to water stress by regulating antioxidant metabolism. This study was designed to examine whether external Ca2+ treatment would improve drought tolerance in liquorice cells. The results showed that water stressed treatment induced by 10% PEG could reduce significantly the FW and RWC of liquorice cells, but external Ca2+ treatment considerably increased the two factors after 10-days stress. In addition, lesser amounts of MDA and H2O2 accumulated in Ca2+-treated cells than in untreated cells, and the activities of CAT, SOD and POD in Ca2+-treated cells were higher than in untreated cells during the stress period. The measured parameters treated by 40 mmol L-1CaCl2 were higher than those treated by 10 mmol L-1CaCl2. The changes in CAT, SOD and POD activities under stressed conditions were significantly larger than those in non-stressed conditions. Under stressed conditions, the trend of SOD activities was similar to that of CAT, and the activity of CAT was larger than that of SOD. CAT activity changed in relation to H2O2 content. It was indicated that water stress induced oxidative stress in liquorice cells, and application of external calcium (40 mmol L-1) significantly improved water stress tolerance in those cells. In addition, the measured parameters were different between Ca2+-treated cells under stressed and non-stressed conditions, and it is possible that calcium signals were different coming from different stimulations. The investigations also showed that the effect of external Ca2+ on the measured parameters was not due to the regulation of osmotic potential and osmotic adjustment in liquorice cells. The mechanism that allowed extracellular Ca2+ to improve adaptation of liquorice cells to drought was mediated by mitigating oxidative stress.
Keywords: Aatioxidant enzyme; Ca2+; Glycyrrhiza uralensis; Polyethylene glycol; Water stress.
Abbreviations: CAT, catalase; FW, fresh weight; MDA, malondialdehyde; PEG, polyethylene glycol; POD, peroxidase; RWC, relative water content; SOD, superoxide dismutase.
Introduction
Liquorice (Glycyrrhiza uralensis Fisch) is a traditional medicinal plant in China. The species has significant abilities to withstand adverse environmental stresses such as drought, cold, and hot. (Qiou et al., 2000; Wang et al., 2001). In addition, liquorice usually grows in rich-calcium soil in arid or semi-arid areas (Qiou et al., 2000; Zhang et al., 2000; Wang et al., 2001), We are thus interested in the correlation between external calcium and water stress tolerance in liquorice.
Drought is a common and serious problem to plants in arid or semi-arid areas. Plants have developed different morphological, physiological, and biochemical mechanisms to withstand drought stress. Evidence from different lines of research suggests that drought stress can induce oxidative stress in plants (Dhindsa and Matowe, 1981; Mukherjee and Choudhwri, 1981; De Lucad'Oro and Trippi,
1987; Trippi et al., 1989). Oxidative stress—resulting from the generation of AOS, such as superoxide (O2-·), peroxide hydrogen (H2O2) and hydroxyl radicals (OH·)—is detrimental to plant survival under a water stress environment. To counteract the toxicity of AOS, a highly efficient antioxidative defense system, including both nonenzymic (e.g. ascorbate, carotenoids, a-tocopherol) and enzymic constituents (e.g. SOD [EC1.15.1.1], POD [EC 1.11.1.7], CAT [EC 1.11.1.6]), is present in plant cells and plays an important role in defending plants from AOS damage (Elstner, 1982; Smirnoff, 1993). The system is able to catalyze or participate in the elimination of free oxygen radicals and H2O2 from cells (Schaedle and Bassham, 1977; Asada and Takahashi, 1987; Wang et al., 1989). Under a drought environment, the balance between the generation and elimination of AOS in plants becomes damaged, causing an accumulation of AOS. Thus, the cell membrane system is destroyed, and a series of metabolite responses causes turbulence (Wang, 1988; Chen, 1989). Plants have the capacity of regulation balance between the generation and elimination of AOS within the range of plants tolerancing adverse environments. Many studies have
*Corresponding author. Fax: +86-931-8912893; E-mail: Wanggx@lzu.edu.cn