Sheue et al. — Resin ducts in Pinus needles
Bot. Bull. Acad. Sin. (2003) 44: 305-313
Altitudinal variation of resin ducts in Pinus taiwanensis Hayata (Pinaceae) needles
Chiou-Rong Sheue1, Yuen-Po Yang1, and Ling-Long Kuo-Huang2,*
1Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung 804, Taiwan
2Department of Life Science, National Taiwan University, Taipei 106, Taiwan
(Received February 13, 2003; Accepted July 31, 2003)
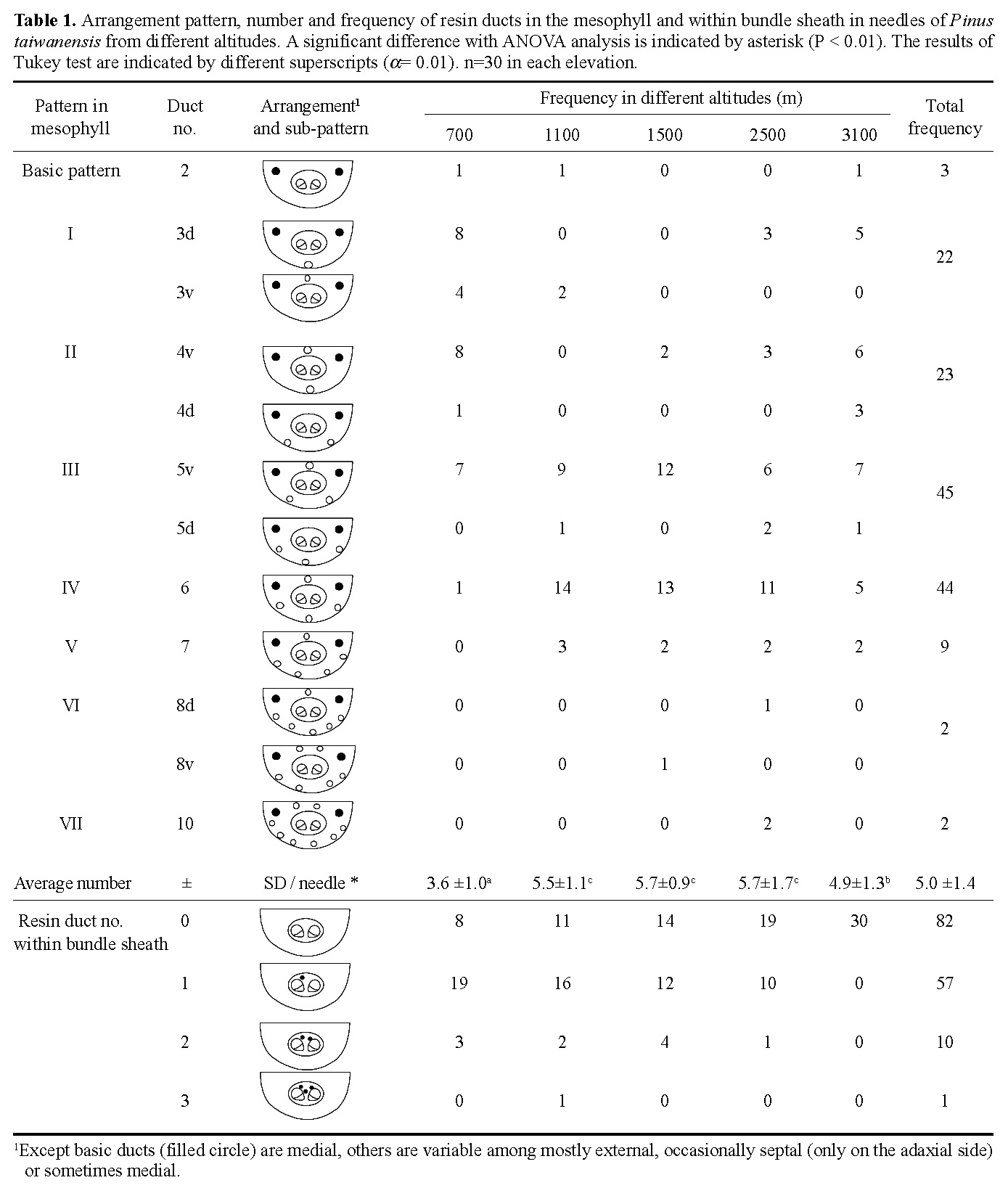
Abstract. The variation of resin ducts in needles of Pinus taiwanensis along an altitude gradient (700 m- 3,100 m) in central Taiwan is revealed for the first time in this study, including position type, number, size, arrangement pattern and anatomical structural variation. Three types of resin ducts-medial, external and septal-were found according to their relative positions in the mesophyll. All needles sampled showed combinations of these three resin duct types. Mostly, 3-7 resin ducts were found in the mesophyll per needle, and the number of resin ducts was significantly greater at mid altitudes than at high and low altitudes. Septal ducts decreased in number with an increase in altitude; however, needles from mid altitudes had more external ducts. Eight patterns (basic and patterns I-VII) of resin ducts in the mesophyll of needles were delineated to present the arrangement variations. In addition to the primary ducts found in the mesophyll, we found the rare bundle sheath ducts in some needles, and their frequencies decreased obviously with an increase in altitude. The consistent combination feature of medial, external, and septal ducts in needles of P. taiwanensis from Taiwan could be easily distinguished from those with medial ducts from mainland China. However, this difference has unrecognized before, and it should be considered in future taxonomic work.
Keywords: Altitudinal variation; Needle; Resin duct; Pinus taiwanensis.
Introduction
In conifers, the resin duct is a common structure of the plant body. It may be present both in the primary and secondary tissues; but it seems to occur more consistently in the former. Previous research has examined the structure, distribution, and development of resin ducts in the Pinaceae (Hanes, 1927; Mergen and Echols, 1955; Werker and Fahn, 1969; Lapasha and Wheeler, 1990; Wu and Hu, 1997).
Napp-Zinn (1966) classified four types of resin ducts according to their position in the needles of Pinus: (1) ducts in contact with the hypodermis (i.e., external); (2) ducts surrounded by chlorenchyma (i.e., medial); (3) ducts in contact with the bundle sheath (i.e., endodermis) in the chlorenchyma (i.e., endonal); and (4) ducts inside the bundle sheath. Although this last type is rare, P. halepensis Mill. is an example of a species possessing bundle sheath resin ducts (Werker and Fahn, 1969). Biswas and Johri (1997) described four similar resin ducts in the mesophyll (medial, external, endonal, and septal) but did not mention Napp-Zinn's fourth type of duct, the one inside the bundle sheath. Most species of Pinus contain one or two types of resin ducts in the needles (Biswas and Johri, 1997).
The resin duct is an important character, applied in classifying the Pineaceae and particularly in distinguishing Pinus species (Law et al., 1978; Li and Keng, 1994; Wu and Hu, 1997; Richardson, 1998; Fu et al., 1999; Boratyńska and Bobowicz, 2001). It is even used to differentiate hybrids (Kormutak et al., 1993). The number and position of resin ducts in needles may vary considerably and interspecifically in pines. Nevertheless, for the identification of pine species, the number of resin canals in the needles is of no particular importance, except for a few species that normally only have two or three canals; however, the relative position of the ducts in the needle may be used as an aid in identification (Harlow, 1931).
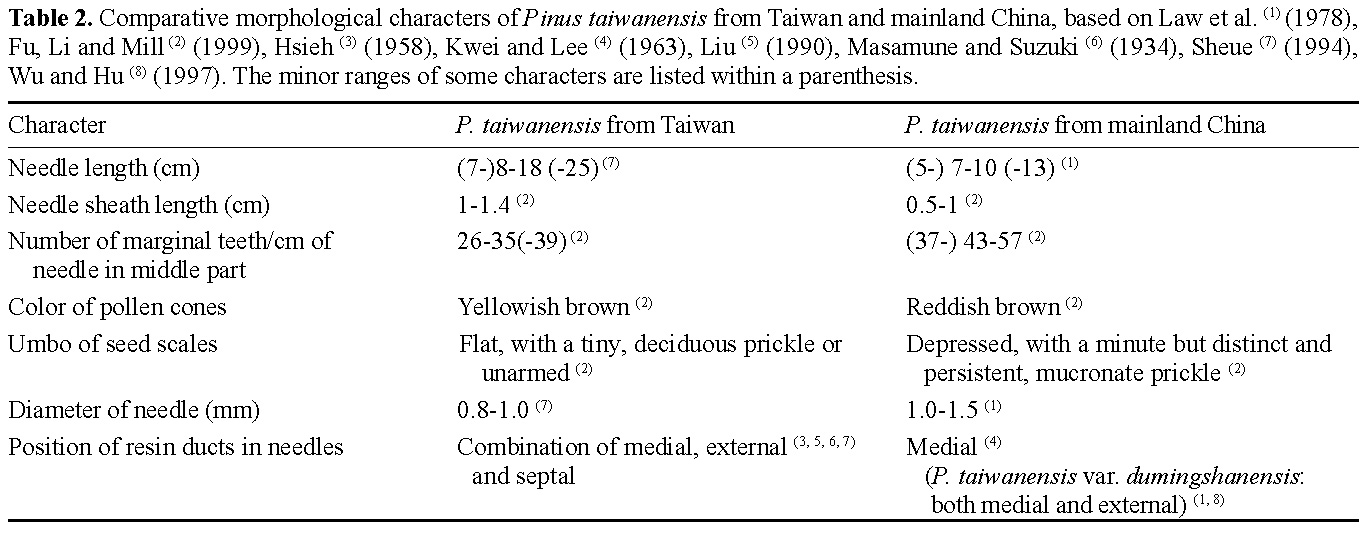
Four species of pines are native to Taiwan. Pinus taiwanensis Hayata of these native species has the widest distribution, ranging from altitudes of 680 m to 3,100 m in central and southern Taiwan (Sheue, 1994). Whether P. taiwanensis is endemic to Taiwan or also occurs in mainland China is hotly debated and depends on clarification of the taxonomic status of P. hwangshanensis Hsia (now treated as a synonym of P. taiwanensis in Flora of China, Fu et al., 1999) (Wu, 1956; Critchfield and Little, 1966; Cheng et al., 1975; Law et al., 1978; Silba, 1984; Li, 1997; Richardson, 1998; Fu et al., 1999). Furthermore, the discrepancy of feature of resin ducts in needles of P. taiwanensis between Taiwan and mainland China is detectable. Resin ducts in needles of so-called P. taiwanensis from mainland China are medial (Kwei and Lee, 1963; Law et al., 1978; Li, 1997; Wu and Hu, 1997) while
*Corresponding author. Tel: 886-2-23630231 ext. 2364; Fax: 886-2-23918940; E-mail: linglong@ccms.ntu.edu.tw