Bot. Bull. Acad. Sin. (2003) 44: 315-321
Yang et al. — Pigment-protein complexes and cecidomyiid gall
Herbivorous insect causes deficiency of pigment-protein complexes in an oval-pointed cecidomyiid gall of Machilus thunbergii leaf
Chi-Ming Yang1,*, Man-Miao Yang2, Jia-Mei Hsu1, and Wann-Neng Jane1
1Institute of Botany, Academia Sinica, Nankang, Taipei, Taiwan 11529
2Department of Entomology, National Chung-Hsing University, 250 Kuo Kuang Rd., Taichung, Taiwan 40227
(Received June 11, 2002; Accepted July 1, 2003)
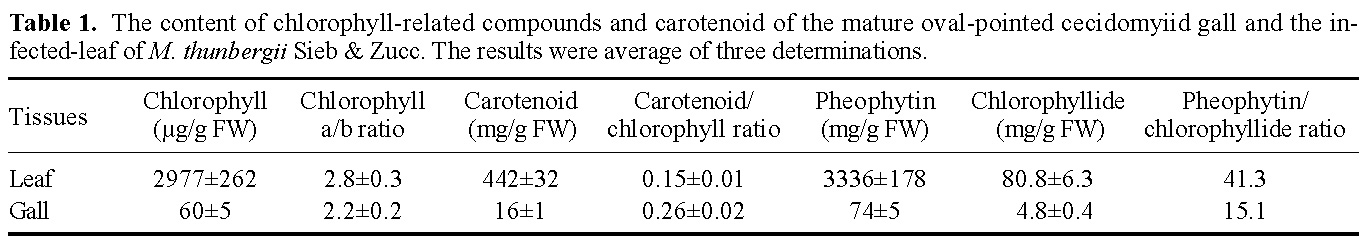
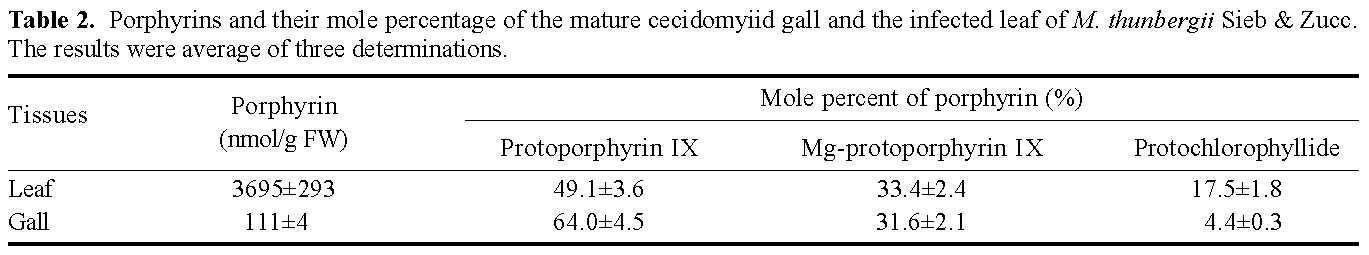
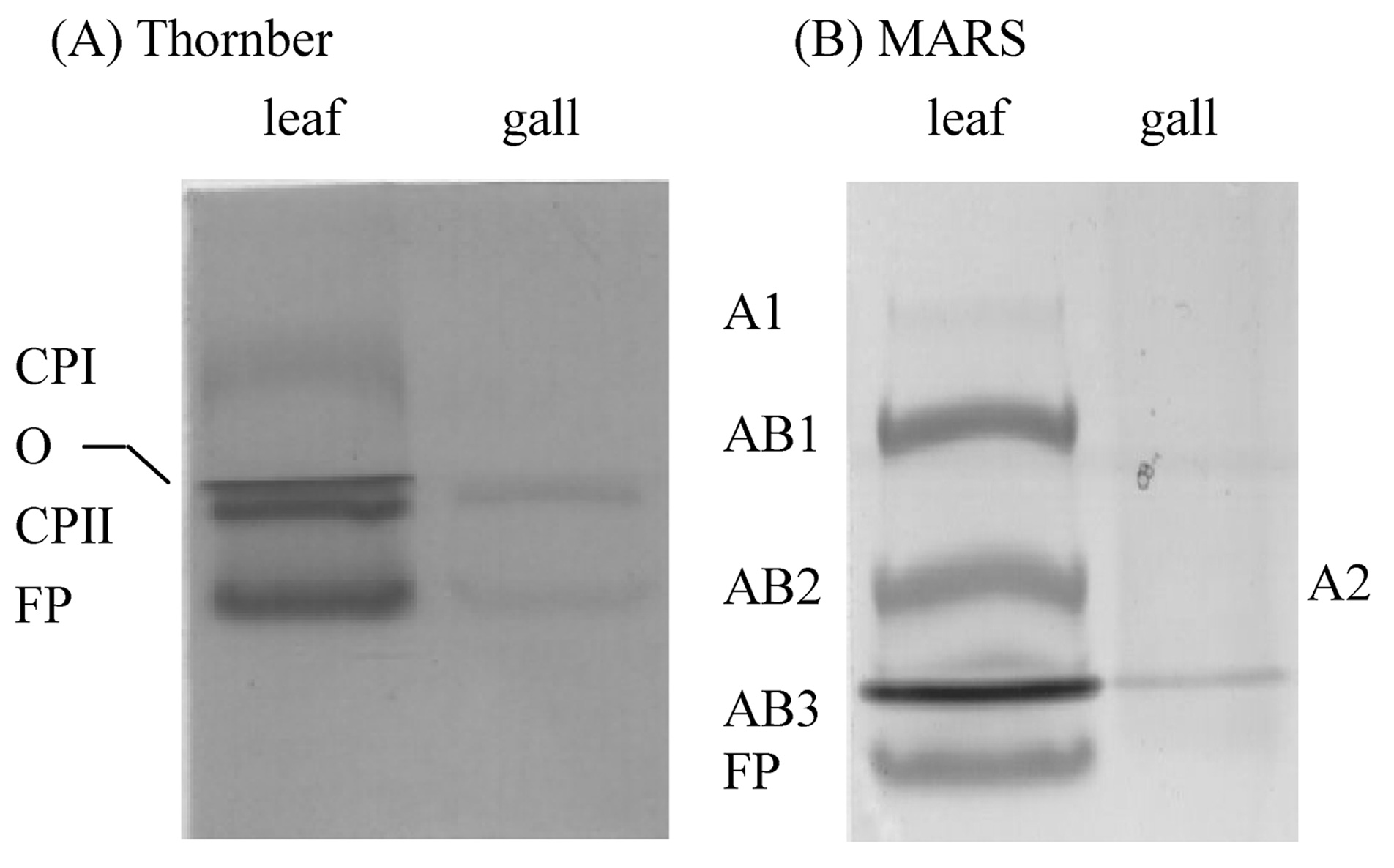
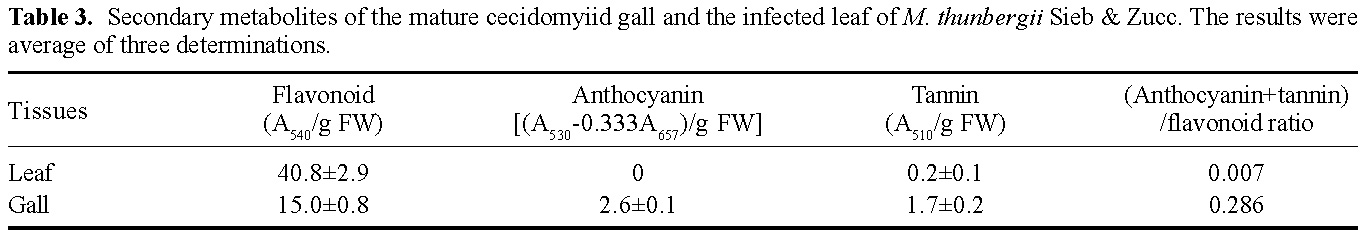
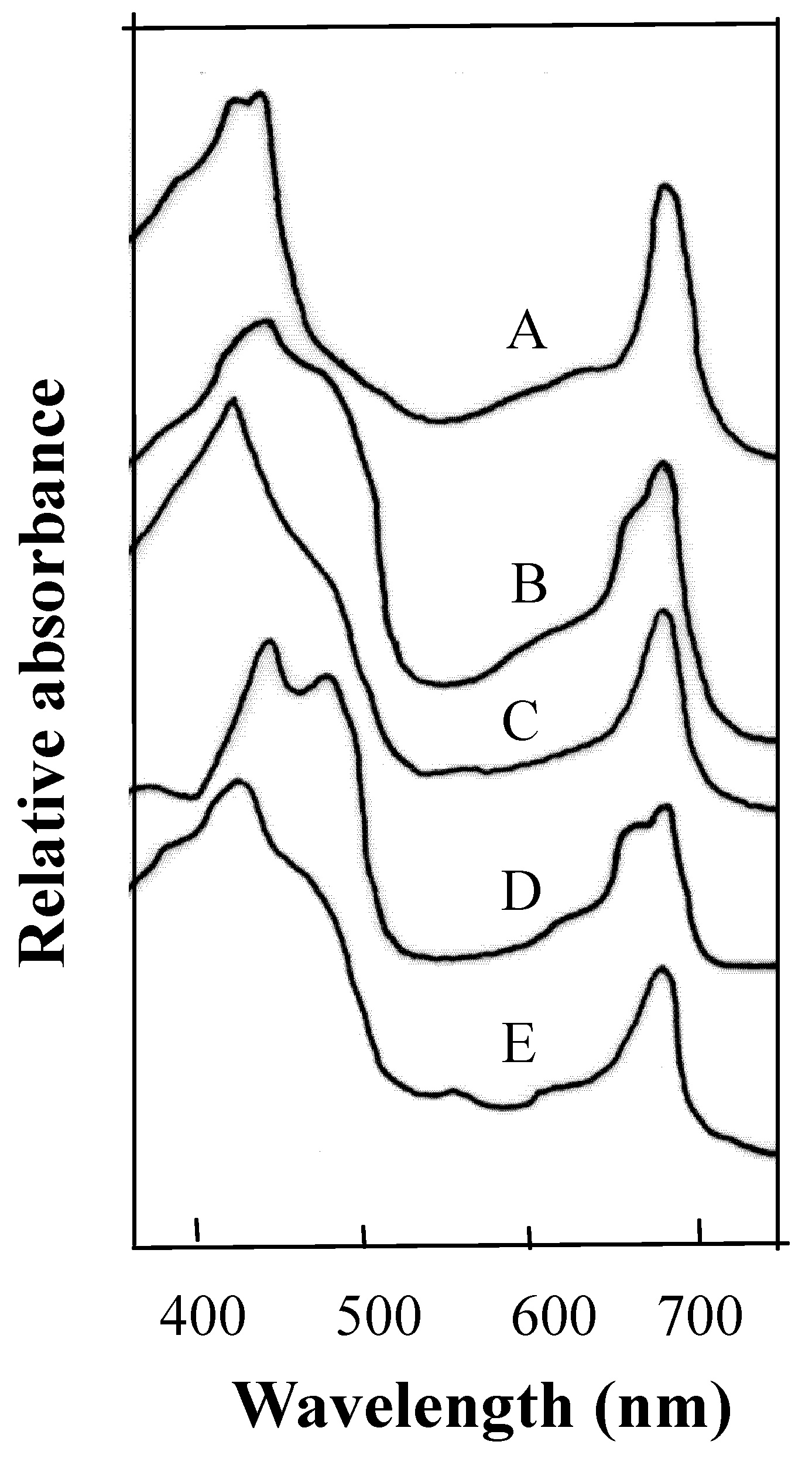
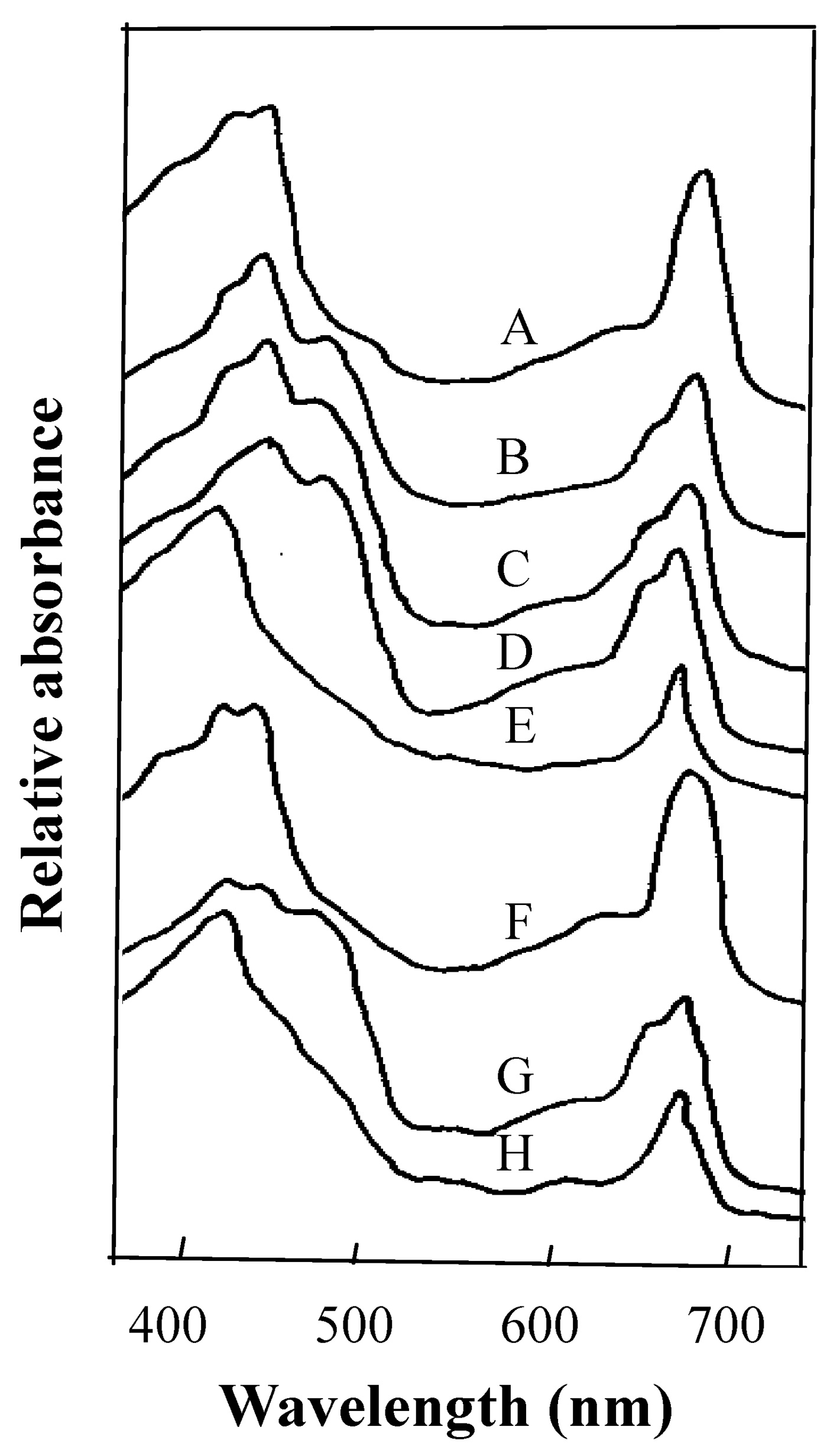
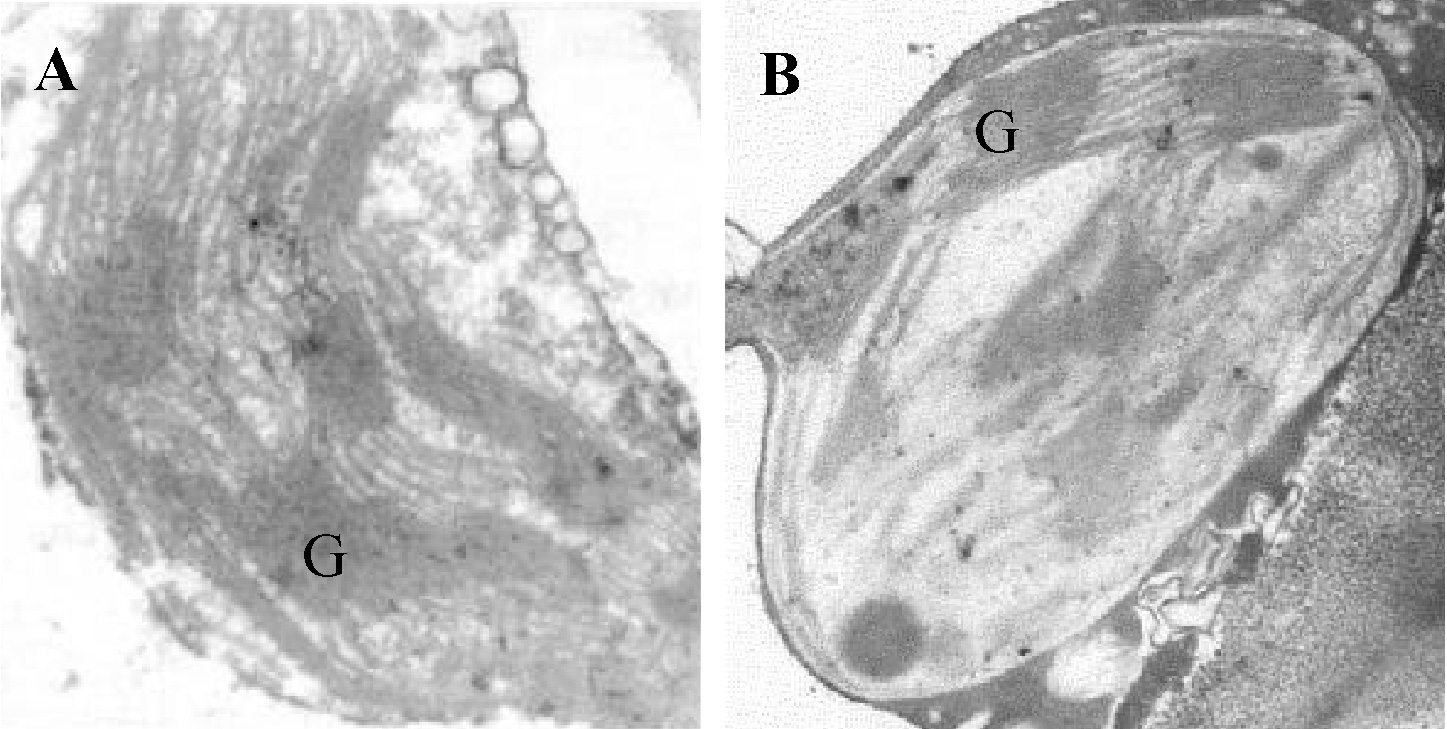
Abstract. This research compared the chlorophyll biosynthetic and degradation pathways, pigment-protein complexes, and thylakoid morphology of a mature oval-pointed cecidomyiid gall and the infected leaf of host plant Machilus thunbergii Sieb & Zucc (Lauraceae). The mature gall always possesses far less photosynthetic pigment than the infected leaf. The content of anthocyanin and tannin of the gall are much higher than in the infected leaf. Both the mole percent of porphyrin and the ratio of pheophytin/chlorophyllide are much different between the gall and infected leaf, suggesting their chlorophyll biosynthetic and degradation pathways are much different. While the infected leaf may take the degradation pathway of chlorophyll®pheophytin®pheophorbide as the major route, the cicedomyiid gall may take chlorophyll®chlorophyllide®pheophorbide as the major route. The infected leaf still possesses the CPI and CPII pigment-protein complexes fractionated by Thornber system, or the A1, AB1, AB2, AB3 pigment-protein complexes fractionated by the MARS system while the mature gall contains only CPII or AB3. Electron microscopy demonstrated that the mature gall has normal grana and thylakoid morphology. It is still unknown whether the unique deficiency of pigment-protein complexes is ubiquitous and how the cecidomyiid insects cause the deficiency of some pigment-protein complexes.
Keywords: Cecidomyiid gall; Herbivorous insect; Machilus thunbergii leaf; Pigment-protein complexes; Thylakoid.
Introduction
Whether induced by viruses, bacteria, fungi, nematodes, mites, or insects via developmental inhibition, differentiation, growth, or suppression of host plant tissues, gall is a well-known type of plant structure and growth form. Multiple changes in response to gall inducers have been found in host plant tissues. These include changes in pH, polarity, nuclear and nucleolar hypertrophy, excess free amino acids and sugars, the presence of hydrolytic enzymes such as amylase and protease, and others (Mani, 1992; Rohfritsch, 1992).
Although all plant organs are subject to insect galls, more than 75% occur on plant leaves (Dreger-Jauffret and Shorthouse, 1992; Yang and Tung, 1998). While much attention has been focused on the morphology and anatomy of insects or their induced galls (Meyer 1987; Dreger-Jauffret and Shorthouse, 1992; Williams, 1994), relatively little work has been done on the chloroplast of galls. The limited reports available about gall chloroplasts all concern the morphology of thylakoid membrane distributed in
the stroma (Rey, 1973, 1974 and 1992). The few studies on gall-former impacts on host photosynthesis that exist do not suggest any general trends, because they report a range of effects from negative to positive (Andersen and Mizell, 1987; Fay et al., 1993; Bagatto et al., 1996; Larson, 1998). It seems that no researcher has studied the biochemical features of thylakoid membrane in the gall chloroplast.
In this study, we therefore analyze for the first time the biochemical composition of pigment-protein complexes of thylakoid membrane isolated from the two cecidomyiid gall chloroplasts of M. thunbergii Sieb & Zucc leaf. A unique pattern of pigment-protein complex different from normal chloroplast was discovered in the gall chloroplast.
Materials and Methods
Plant and Gall
The mature oval-pointed cecidomyiid galls (Figure 1) residing on the lower epidermis of Machilus thunbergii Sieb & Zucc. (Lauraceae) mature leaf was collected from Chung-Cheng Mountain of the Yang-Ming Shan National Park in northern Taiwan. The mature galls were detached from the infected mature leaf, and the surrounding healthy leaf tissue was trimmed to avoid contamination.
*Corresponding author. Tel: 886-2-27821258 ext. 612; Fax: 886-2-27827954; E-mail: cmyang@gate.sinica.edu.tw