Bot. Bull. Acad. Sin. (2004) 45: 41-48
Hsu and Kao Abscisic acid and phosphinothricin-tolerant rice seedlings
Phosphinothricin tolerance in rice (Oryza sativa L.) seedlings is associated with elevated abscisic acid in the leaves
Yi Ting Hsu and Ching Huei Kao*
Department of Agronomy, National Taiwan University, Taipei, Taiwan, Republic of China
(Received March 18, 2003; Accepted August 29, 2003)
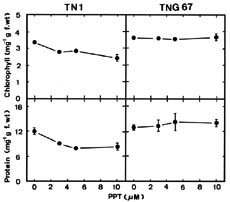
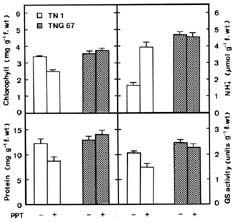
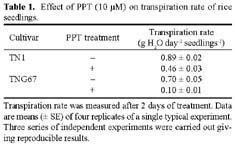
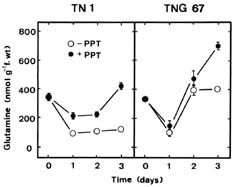
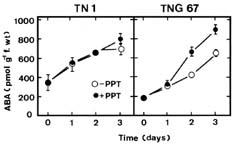
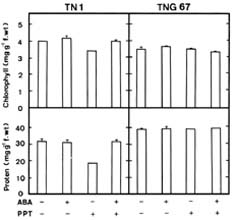
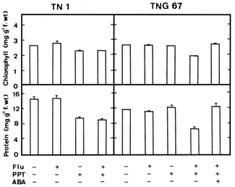
Abstract. Phosphinothricin (PPT, known as glufosinate) is a nonselective herbicide that is a potent inhibitor of glutamine synthetase (GS). PPT-tolerant and PPT-sensitive rice cultivars were used to study the role of abscisic acid (ABA) in PPT tolerance. ABA content was determined by enzyme-linked immunosorbent assay. PPT toxicity was evaluated by the decrease in chlorophyll and protein contents in the leaves. On treatment with PPT, ABA content significantly increased in the leaves of the PPT-tolerant cultivars (cv. Tainung 67, TNG67) but not in the PPT-sensitive cultivar (cv. Taichung Native 1, TN1). The reduction of transpiration rate of TN1 seedlings caused by PPT was less than that of TNG67. Pretreatment with ABA enhanced PPT tolerance and reduced PPT-induced NH4+ accumulation in leaves of TN1. Exogenous application of the ABA biosynthesis inhibitor, fluridone, decreased PPT tolerance and increased NH4+ content in the leaves of TNG 67. Fluridone effect on PPT toxicity of TNG67 seedlings was reversed by the application of ABA. Evidence is presented to show that PPT-induced NH4+ accumulation, rather than depletion of glutamine, is the mechanism that regulates PPT-induced toxicity in TN1 leaves. In conclusion, the increase in endogenous ABA content in the leaves is closely related to PPT tolerance of rice seedlings.
Keywords: Abscisic acid; Ammonium ion toxicity; Glutamine synthetase; Phosphinothricin tolerance; Rice.
Abbreviations: ABA, abscisic acid; ELISA, enzyme-linked immunosorbent assay; f.wt, fresh weight; GS, glutamine synthetase; PPT, phosphinothricin; TN1, Taichung Native 1; TNG67, Tainung 67.
Introduction
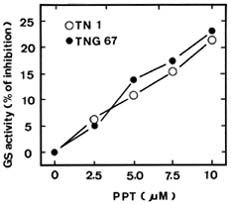
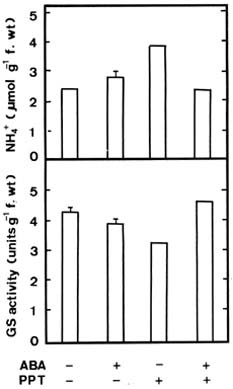
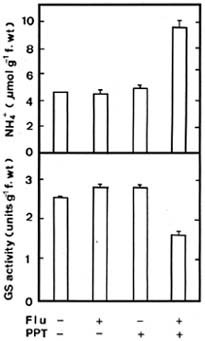
Glutamine synthetase (GS, EC 6.3.1.2) is the initial enzyme in the pathway that assimilates inorganic nitrogen into organic compounds (Lea and Miflin, 1974). It is an important enzyme in nitrogen metabolism in that, in addition to assimilating NH4+ produced by nitrite reductase, reassimilates NH4+ released as a result of photorespiration and the breakdown of proteins and nitrogen transport compounds (Miflin and Habash, 2002). Phosphinothricin [PPT, 2-amino-4-(methylphosphinyl)-butanoic acid, known as glufossinate] is a nonselective herbicide that is a potent inhibitor of GS (Leason et al., 1982; Ridley and McNally, 1985). Inhibition of GS activity by PPT leads to a rapid accumulation of NH4+ under conditions in which nitrite is being photosynthetically reduced and/or conditions which support photorespiration (Tachinbana et al., 1986; Wild et al., 1987; Sauer et al., 1987; Wendler et al., 1990). A high level of NH4+ is known to have a toxic effect on plant cells (Givan, 1979). Thus, PPT toxicity may be a direct result of NH4+ accumulation.
The plant hormone abscisic acid (ABA) is a sesquiterpenoid synthesized from xanthophylls (Taylor et al., 2000; Seo and Koshiba, 2002) and appears to influence several physiological and developmental events (Zeevaart
and Creelman, 1988; Kende and Zeevaart, 1997). The level of ABA in plants increases upon their exposure to environmental stress (Zeevaart and Creelman, 1988; Xu et al., 1995). ABA content has been correlated with increased tolerance to freezing (Guy, 1990), chilling (Lee et al., 1993), drought (Zeevaart and Creelman, 1988), salt (LaRose et al., 1987), and cadmium toxicity (Hsu and Kao, 2003).
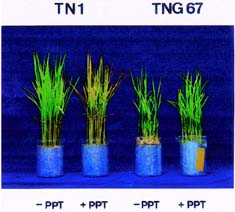
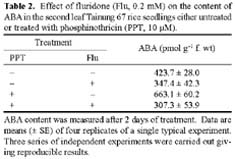
It has been shown that ABA accumulation is a common effect of using auxin herbicides on susceptible plants (Grossmann et al., 1996; Grossmann, 2000; Hansen and Grossmann, 2000). Recently, we demonstrated that PPT treatment resulted in an increase in ABA level in detached rice leaves (Tsai et al., 2002). However, no correlation between ABA and PPT toxicity or PPT tolerance in detached rice leaves could be established (Tsai et al., 2002). Our preliminary observation showed that rice seedlings of cultivar Tainung 67 (TNG67) are more tolerant to PPT than those of cultivar Taichung Native 1 (TN1) when PPT was added directly to the culture solution. It appears that these two cultivars of rice seedlings with different tolerance to PPT provide a useful system to study mechanism of PPT tolerance of rice plants. In this investigation, we examine the role of ABA in PPT tolerance of rice seedlings.
Materials and Methods
Plant Cultivation and Treatment
Two rice (Oryza sativa L.) cultivars, an Indica type cultivar TN1 and a Japonica cultivar, TNG67, obtained from
*Corresponding author. Tel: 886-2-23698159; Fax: 886-2-23620879; E-mail: kaoch@ntu.edu.tw