Shangguan et al. — Nitrogen on root and gas exchange
Bot. Bull. Acad. Sin. (2004) 45: 49-54
Effect of nitrogen on root and shoot relations and gas exchange in winter wheat
Z.P. Shangguan1,2,*, M.A. Shao1,2, S.J. Ren2, L.M. Zhang2, and Q. Xue3
1National Laboratory of Soil Erosion and Dryland Agriculture on the Loess Plateau, Institute of Soil and Water Conservation, Chinese Academy of Sciences, Yangling, Shaanxi Province, 712100, P.R. China
2Northwest Sci-Tech University of Agriculture and Forestry, Yangling, Shaanxi Province, 712100, P.R. China
3Northwestern Agricultural Research Center, Montana State University, 4570 Montana 35, Kalispell, MT 59901, USA
(Received April 8, 2003; Accepted July 4, 2003)
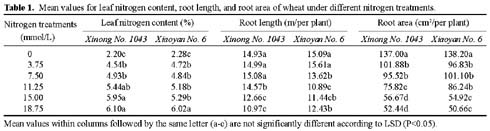
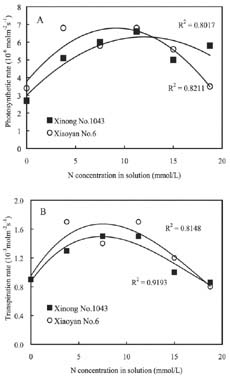
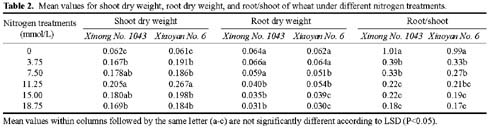
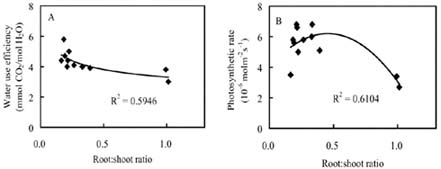
Abstract. The seedling growth of two drought-resistant wheat varieties was studied under solution culture in a plant growth chamber. The results showed that the shoot dry weight and leaf gas exchange parameters increased with the increase of nitrogen supply, but decreased when nitrogen supply reached a certain level. The optimum nitrogen concentrations for shoot dry weight and gas exchange were different among the varieties. The root growth was negatively correlated with the increase of nitrogen supply. The distribution of root length in different layers was similar for the two varieties. The root length was the longest at the layer of 5-15 cm, the shortest below 15 cm, and in between at the layer of 0-5 cm. The water use efficiency (WUE) decreased with increasing ratio of root to shoot (R/S), while leaf photosynthetic rate tended to increase initially and then decrease. The increase in R/S was unfavorable to increase WUE, and the appropriate R/S for leaf photosynthetic rate was about 0.5.
Keywords: Coordinate growth; Nitrogen nutrition; Root and shoot relation; Winter wheat.
Introduction
Drought stress and nitrogen deficiency are major constraints to winter wheat production and yield stability in most rainfed regions (McDonald and Davies, 1996). An efficient use of limited nitrogen resources and better growth under limited nitrogen supply are desirable traits for crops in drought environments. Nitrogen deficiency induces modifications of many morphological and physiological processes (Ciompi et al., 1996; Shangguan et al., 2000a). Leaf nitrogen is mostly used for synthesis of components of the photosynthetic apparatus, and about 75% of leaf nitrogen is allocated to the chloroplasts (Shangguan et al., 2000b).
The nutrient supply and demand of root and shoot are inter-dependent due to their different functions and local environment (Passioura, 1983; Siddique et al., 1990; Li et al., 2001). The ratio of root to shoot (R/S) is an index that reflects growth and dry matter accumulation between root and shoot (Lioert et al., 1999). The R/S is affected both by genetic (Passioura, 1983; O'Toole and Bland, 1987) and environmental factors, such as water status (Miao et al., 1998; Grant, 1998; Hebert et al., 2001), nutrient availability (Liang, 1996; Marsh and Pierzynski, 1998; Maranov et al., 1998), and soil texture (Vos et al., 1998). Root growth is
closely related to physiological metabolism and dry matter accumulation in shoot (Siddique et al., 1990). An excessively low R/S indicates poor root growth, resulting in sufficient water and nutrients for shoot growth. An extremely high R/S may lead to root redundancy, which reduces shoot growth, yield, and water and nutrient use efficiencies (Zhang, 1995). Therefore, it is important to coordinate root and shoot relations and maximize dry matter accumulation and water and nutrient use efficiencies (Tomar et al., 1997; Kahn and Schroeder, 1999).
Nitrogen nutrition has significant effects on root and shoot relations (Feng and Liu, 1996; Lioert et al., 1999). Nitrogen deficiency increased root surface area, increased consumption of assimilates, reduced the amount of nitrogen transported to shoot, decreased shoot growth, and resulted in an increased R/S ratio. However, extra nitrogen nutrition caused an excessive shoot growth, reduced the assimilate availability for root, and reduced the R/S ratio (Passioura, 1983). Therefore, the amount of nitrogen nutrition applied to plants must be optimal for root and shoot relations. Previous studies have shown that effective use of nitrogen fertilizer increased leaf photosynthesis, promoted root development, and extended space for root to extract water and nutrients in soil (Li et al., 1999). However, related research is relatively scarce, and then the effect of nitrogen nutrition on root growth needs to be enhanced. Objectives of this study were to (1) investigate the responses of shoot and root growth, leaf exchange, and WUE to nitrogen nutrition and (2) determine the relationships between gas exchange, WUE and R/S.
*Corresponding author. Tel: +86-29-7019107; Fax: +86-29-7012210; E-mail: shangguan@ms.iswc.ac.cn