Bot. Bull. Acad. Sin. (2004) 45: 69-85
Jane —Ultrastructure of endosperm development in Arundo formosana
Ultrastructure of endosperm development in Arundo formosana Hack. (Poaceae) from differentiation to maturity
Wann-Neng Jane
Electron Microscopy Lab, Institute of Botany, Academia Sinica, Taipei, Taiwan
(Received January 7, 2003; Accepted August 27, 2003)
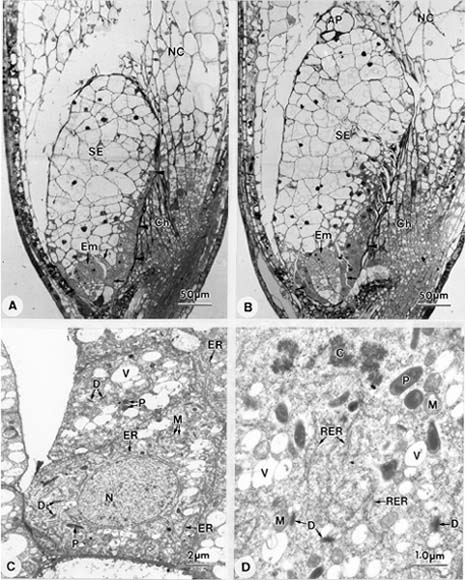
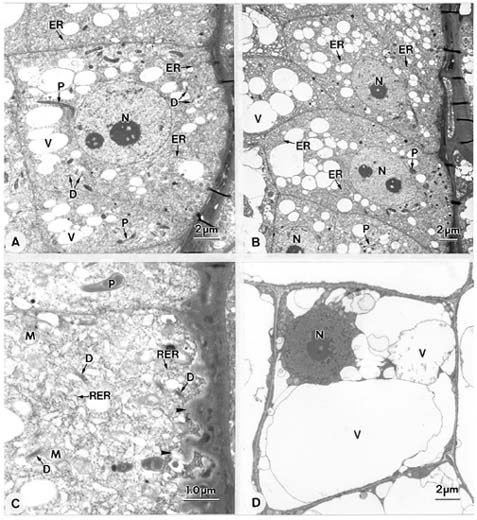
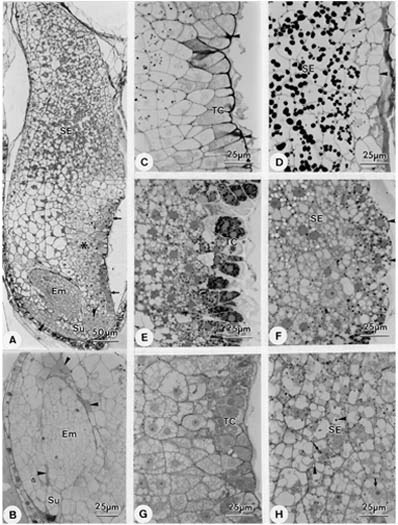
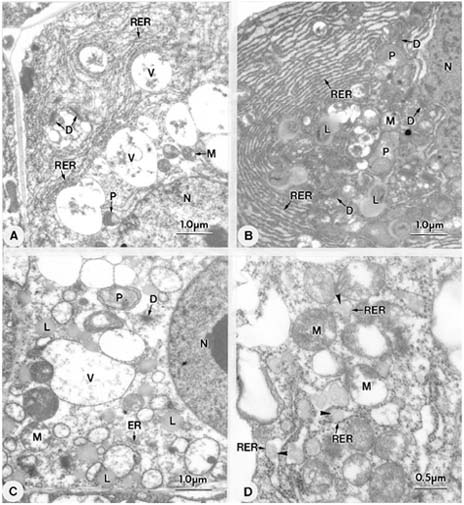
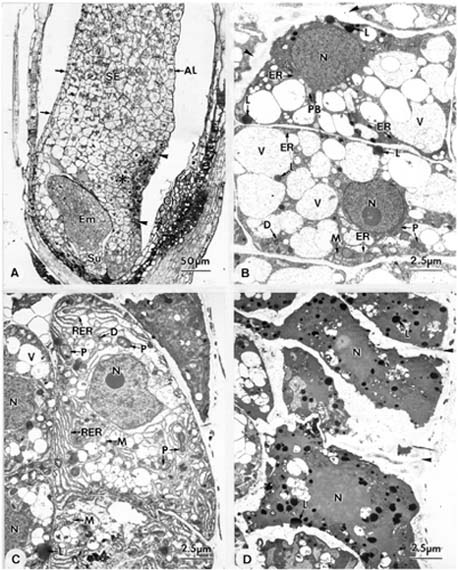
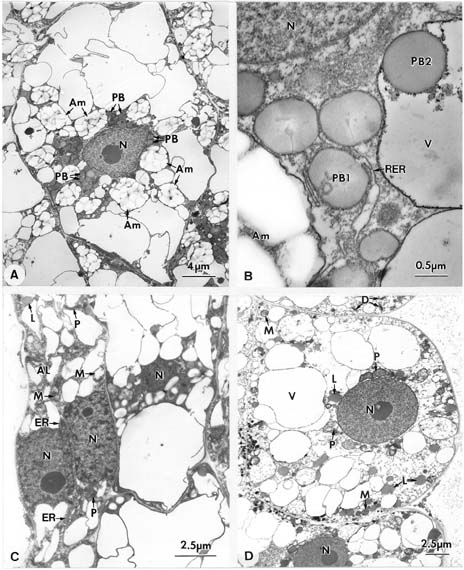
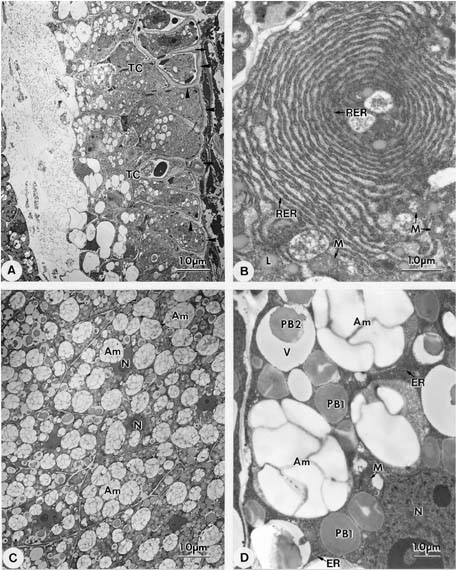
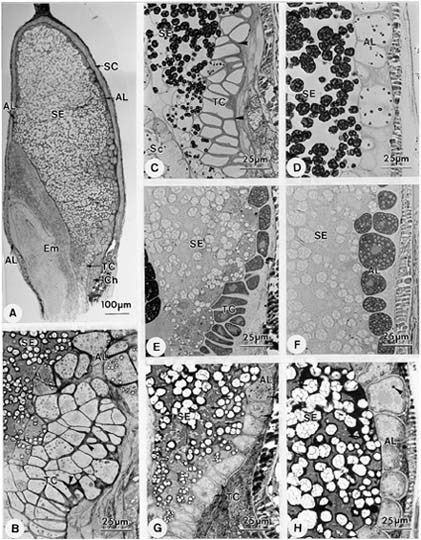
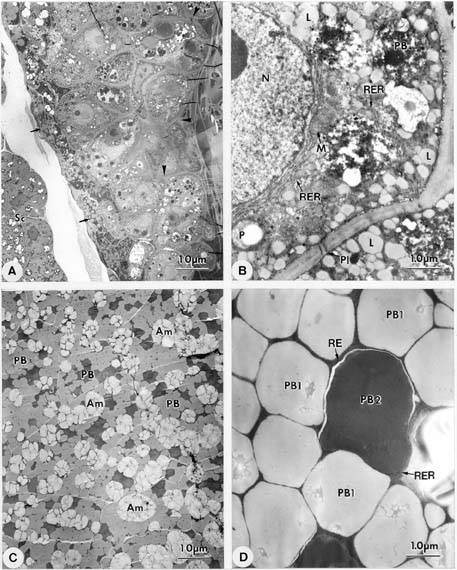
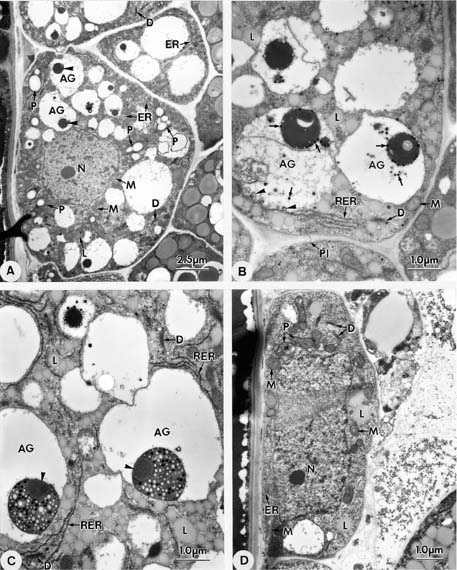
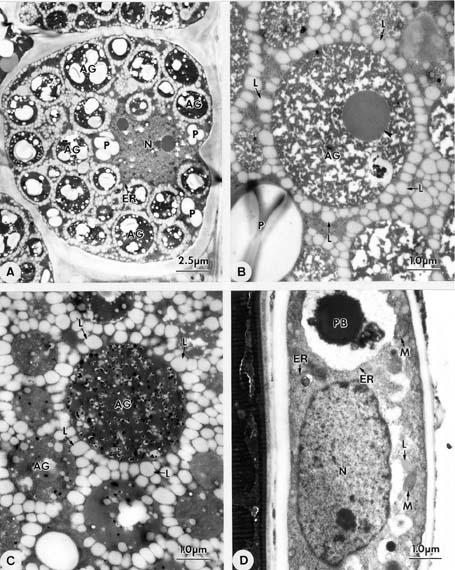
Abstract. Endosperm development of Arundo formosana Hack. is examined ultrastructurally and histochemically from differentiation to seed maturity. The differentiated endosperm contains four major cell types: the cells of the embryo surrounding region, transfer cells, aleurone layer, and starchy endosperm. After cellularization, cells of the embryo surrounding region and transfer cells contain dense cytoplasm and large quantities of rough endoplasmic reticulum (RER) and many dictyosomes. In the transfer cells, the wall ingrowths are present on the walls adjacent to the nucellus. The embryo surrounding region is distributed on the ventral side and surrounds the suspensor. After leaf primordium initiation, most cells of the embryo surrounding region have degenerated, but the outermost layer of the ventral side becomes part of the aleurone layer. The transfer cells differentiate into outer and inner cells. The outer transfer cells contain electron-dense cytoplasm, many oil bodies, large quantities of RER, and dictyosomes. These cells appear PAS-positive, especially in the thickening walls. The inner transfer cells have electron-transparent cytoplasm, some small vacuoles, organelles, and oil bodies. The outer transfer cells are finally compressed and degenerated. The inner cells contain PAS-positive thickening walls, many oil bodies, protein bodies, and RER at maturity. The starchy endosperm cells gradually accumulate reserves (mainly proteins and starch) during development, and are filled with amyloplasts and protein bodies at maturity. The protein bodies are of two types that originate in the RER and are then stored in the cisternal lumen of RER, and vacuoles, respectively. The outermost layer of the endosperm becomes the aleurone layer, except for the transfer cell region. The contents of the aleurone cells are initially similar to other starchy endosperm cells, and are finally filled with aleurone grains surrounded by many small lipid bodies. The aleurone grains are initiated from ER and accumulate in vacuoles.
Keywords: Aleurone grain; Aleurone layer; Arundo formosana Hack.; Embryo surrounding region; Endosperm development; Protein body; Starchy endosperm; Transfer cell.
Introduction
A general model for the development of cereal endosperm (Olsen et al., 1995; Olsen et al., 1998; Olsen et al., 1999) recognizes four major stages: syncytial, cellularization, differentiation, and maturation. After cellularization, the endosperm differentiates into two cell types in Triticum (Smart and O'Brien, 1983) and Zea (Schel et al., 1984), one with dense cytoplasm and the other with large vacuoles. Smart and O'Brien (1983) termed the former modified endosperm and the later general endosperm. Large quantities of RER are characteristic of the modified endosperm. The wall ingrowths occur in the placentochalazal region of Zea (Schel et al., 1984), but not in that of Triticum (Smart and O'Brien, 1983). Beyond the absorption and transport function of the endosperm, a high degree of synthesis also takes place in the modified endosperm (Schel et al., 1984).
According to the mechanisms of nuclear endosperm cellularization and development in cereals, Olsen et al.
(1999) and Olsen (2001) suggested that the differentiated cereal endosperm is composed of four cell-types: the embryo surrounding region, transfer cells, the starchy endosperm, and the aleurone layer. The underlying genetic programs for cell fate specification probably originated as independent genetic programs (Olsen, 2001).
In a previous paper, I described the embryo development of Arundo in detail (Jane, 1999). In this study, I will observe endosperm development from differentiation to maturation and discuss the relationship of endosperm and embryo development.
Material and Methods
Grains (caryopses) of Arundo formosana Hack., an endemic grass in Taiwan, were collected from Pingshi and Wulai of Taipei county. The voucher specimens, identification number 229386-229391, are deposited in the Herbarium of Department of Botany, National Taiwan University. Whole grains were fixed in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.2 at 4°C overnight. After three 20 min buffer rinses, the grains were postfixed in 1% OsO4 in the same buffer for 4 h at room temperature and then rinsed in three 20 min changes of buffer. The
*Corresponding author. Tel: 886-2-27899590 ext.110; Fax: 886- 2-27827954; E-mail: wnjane@gate.sinica.edu.tw